作为过一硫酸盐创新活化剂的钼烯装饰尖晶石氧化物可用于降解污水处理厂废水中的咖啡因:对机理的见解
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
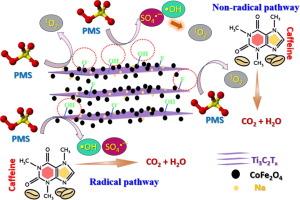
在与废水处理效率相关的环境问题方面,利用二维材料生成高级氧化过程(AOPs)似乎是最有前途的解决方案之一。本研究设计了一种新型过一硫酸盐活化(PMS)催化系统,该系统以装饰有尖晶石氧化物 Co3O4、Fe3O4 和 CoFe2O4 催化剂的 MXene(Ti3C2Tx)为基础。评估了它们通过 PMS 活化降解咖啡因(CAF)的效率。SEM 和 TEM 分析表明,尖晶石氧化物插入了 MXene 的多层结构中,并进行了均匀的表面装饰,这也避免了磁性颗粒的聚集,从而提高了它们的效率。在不同的催化剂中,MXene/CoFe2O4(MXCF)最为有效,这主要归功于铁和钴的氧化还原循环。使用 0.2 g/L 的 MXCF 和 0.5 mM 的 PMS,在自然 pH 值条件下,CAF 在黑暗中 10 分钟内即可完全降解。本研究的新颖之处在于首次利用 MXCF 在黑暗中有效地活化了 PMS,并从机理上阐明了 PMS 的活化过程。研究强调了 Co3+/Co2+ 和 Fe3+/Fe2+ 氧化还原循环以及表面结合官能团的重要作用。自由基清除和 EPR 实验证实 -OH 和 1O2 是参与 CAF 降解的主要 ROS。CAF 的降解途径指向羟基化和咪唑开环机制,而 MXCF 催化剂在通过 PMS 活化降解磺胺甲噁唑和苯酚方面也表现出很高的效率。为了进一步突出所获结果的相关性,在处理布拉迪斯拉发废水处理厂(WWTP)受 CAF 污染的三级出水时,在黑暗条件下加入 0.2 克/升催化剂和 2 毫摩尔 PMS,3 小时后污染物完全降解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mxene-decorated spinel oxides as innovative activators of peroxymonosulfate for degradation of caffeine in WWTP effluents: Insights into mechanisms
In the frame of the environmental issues related to the efficiency of wastewaters treatment, the generation of advanced oxidation processes (AOPs) by 2D materials appears one of the most promising solutions. In this study, a novel catalytic system for peroxymonosulfate activation (PMS) was designed based on MXene (Ti3C2Tx) decorated with spinel oxides Co3O4, Fe3O4 and CoFe2O4 catalysts. Their efficiency in caffeine (CAF) degradation via PMS activation was assessed. The insertion of spinel oxides inside the multilayer structure of MXene along with their uniform surface decoration was demonstrated by SEM and TEM analyses and it also avoided the aggregation of the magnetic particles, thus increasing their efficiency. Among the different catalysts, the MXene/CoFe2O4 (MXCF) stood out as the most effective, mainly due to the Fe and Co redox cycles. The complete degradation of CAF was achieved in the dark within 10 min at natural pH using 0.2 g/L of MXCF and 0.5 mM of PMS. The novelty of current study lies in the efficient activation of PMS by, for the first time, MXCF in the dark along with mechanistic elucidation of PMS activation. The important role of Co3+/Co2+ and Fe3+/Fe2+ redox cycles alongside surface bound functional groups were highlighted. Radical scavenging and EPR experiments confirmed •OH and 1O2 as the main ROS involved in the CAF degradation. The CAF degradation pathways pointed to hydroxylation and imidazole ring opening mechanisms and MXCF catalyst also exhibited high efficiency in the degradation of sulfamethoxazole and phenol via PMS activation. To further highlight the relevance of the obtained results, treatment of tertiary effluents of wastewaters treatment plant (WWTP) in Bratislava contaminated by CAF exhibited a complete pollutant degradation after 3 h by supplying 0.2 g/L of catalyst and 2 mM PMS in the dark.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


