利用基于纳米气泡的对比增强超声成像评估肿瘤中治疗纳米粒子的聚集情况
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究探讨了以纳米粒子为基础向肿瘤实质给药所面临的挑战,重点是广泛使用的增强渗透性和滞留效应(EPR)。虽然 EPR 一直是一种关键策略,但其不一致的临床成功缺乏明确的机理认识,并受到研究相关现象的有限工具的阻碍。这项工作介绍了一种采用纳米级造影剂的多参数动态对比增强超声(CEUS)方法,用于无创、实时检查肿瘤微环境特征。我们证明 CEUS 成像可以(1) 评估肿瘤微环境特征;(2) 帮助预测负载多柔比星的脂质体在肿瘤实质中的分布;(3) 预测纳米治疗效果。利用纳米气泡(NBs)对高(LS174T)和低(U87)血管通透性的两种肿瘤进行了 CEUS 分析。与 U87 肿瘤相比,LS174T 肿瘤始终显示出明显不同的时间强度曲线(TIC)参数,包括上升曲线下面积(AUCR,2.7 倍)和达到峰值强度的时间(TTP,1.9 倍)。最重要的是,最近开发的专门用于 NB CEUS 动态的去相关时间 (DT) 参数成功预测了多柔比星脂质体在肿瘤实质内的分布(r = 0.86 ± 0.13)。在SKOV-3肿瘤中,AUCR、TTP和DT用于将成像结果与纳米治疗反应相关联,准确率达100%。这些研究结果表明,NB-CEUS参数能有效鉴别肿瘤血管通透性,可作为识别肿瘤特征和预测纳米颗粒疗法反应性的生物标记物。在 LS174T 和 U87 肿瘤中观察到的差异以及在 SKOV-3 肿瘤中对纳米疗法疗效的准确预测表明,这种方法在预测疗效和评估以病理通透性血管为特征的疾病的 EPR 方面具有潜在的实用性。最终,这项研究为完善给药策略和评估基于 EPR 方法的更广泛适用性提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
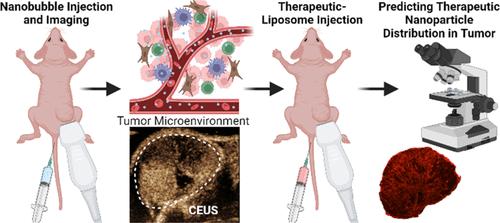
Assessing Therapeutic Nanoparticle Accumulation in Tumors Using Nanobubble-Based Contrast-Enhanced Ultrasound Imaging
This study explores the challenges associated with nanoparticle-based drug delivery to the tumor parenchyma, focusing on the widely utilized enhanced permeability and retention effect (EPR). While EPR has been a key strategy, its inconsistent clinical success lacks clear mechanistic understanding and is hindered by limited tools for studying relevant phenomena. This work introduces an approach that employs multiparametric dynamic contrast-enhanced ultrasound (CEUS) with a nanoscale contrast agent for noninvasive, real-time examination of tumor microenvironment characteristics. We demonstrate that CEUS imaging can: (1) evaluate tumor microenvironment features, (2) be used to help predict the distribution of doxorubicin-loaded liposomes in the tumor parenchyma, and (3) be used to predict nanotherapeutic efficacy. CEUS using nanobubbles (NBs) was carried out in two tumor types of high (LS174T) and low (U87) vascular permeability. LS174T tumors consistently showed significantly different time intensity curve (TIC) parameters, including area under the rising curve (AUCR, 2.7×) and time to peak intensity (TTP, 1.9×) compared to U87 tumors. Crucially, a recently developed decorrelation time (DT) parameter specific to NB CEUS dynamics successfully predicted the distribution of doxorubicin-loaded liposomes within the tumor parenchyma (r = 0.86 ± 0.13). AUCR, TTP, and DT were used to correlate imaging findings to nanotherapeutic response with 100% accuracy in SKOV-3 tumors. These findings suggest that NB-CEUS parameters can effectively discern tumor vascular permeability, serving as a biomarker for identifying tumor characteristics and predicting the responsiveness to nanoparticle-based therapies. The observed differences between LS174T and U87 tumors and the accurate prediction of nanotherapeutic efficacy in SKOV-3 tumors indicate the potential utility of this method in predicting treatment efficacy and evaluating EPR in diseases characterized by pathologically permeable vasculature. Ultimately, this research contributes valuable insights into refining drug delivery strategies and assessing the broader applicability of EPR-based approaches.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


