在三苯基苯-二甲氧基对苯二甲醛-共价有机框架-Mo 上进行光催化 Achmatowicz 重排,将生物质衍生的糠醇转化为氢化吡喃酮
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
将以生物质为基础的原料转化为高价值的平台化合物仍然是一个基本但具有挑战性的课题。由于生物质的复杂性和有限的升级策略,有关此类转化的研究很少。光催化有氧氧化是利用生物质衍生化学品制造药物前体的一种前景广阔的途径。在本研究中,我们通过单线态氧(1O2)介导的光催化 Achmatowicz 反应,展示了利用钼掺杂的三苯基苯二甲氧基对苯二甲醛共价有机框架将糠醇转化为二氢吡喃酮乙酰的过程。COF-Mo 光催化剂在生成羟基-2H-吡喃-3(6H)-酮(一种对合成抗艾滋病药物至关重要的复合合成酮)方面具有高达 98% 的优异选择性。钼的加入使生成率提高了 3.9 倍,这主要归功于钼活性位点与 COF 骨架之间的协同效应。更重要的是,Mo 团簇为 COF 与吸附的 O2 之间的光生电子传递创造了一个超快的电荷隧道。钼的金属态和六价分别促进了超氧阴离子和 1O2 的生成,从而促进了光催化反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
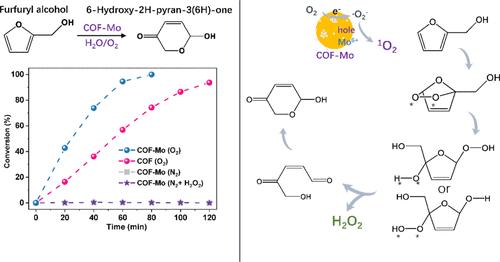
Photocatalytic Achmatowicz Rearrangement on Triphenylbenzene–Dimethoxyterephthaldehyde–Covalent Organic Framework-Mo for Converting Biomass-Derived Furfuryl Alcohol to Hydropyranone
The conversion of biomass-based feedstocks into high-value platform compounds remains a fundamental but challenging topic. Studies on such transformations are sparse due to the complex nature of biomass and limited upgrading strategies. Photocatalytic aerobic oxidation is a promising pathway for making pharmaceutical precursors from biomass-derived chemicals. In this study, we demonstrate the transformation of furfuryl alcohol into dihydropyranone acetyl by using a molybdenum-incorporated triphenylbenzene–dimethoxyterephthaldehyde–covalent organic framework via a singlet oxygen (1O2)-mediated photocatalytic Achmatowicz reaction. The COF-Mo photocatalyst exhibits exceptional selectivity up to 98% in producing hydroxy-2H-pyran-3(6H)-one, a complex synthone crucial for synthesizing anti-AIDS drugs. Mo incorporation enhances the generation rate by 3.9 times, primarily due to the synergistic effect between Mo active sites and the COF backbone. More importantly, Mo clusters create a superfast charge tunnel for photogenerated electron transfer from COF to adsorbed O2. The metallic state and hexavalent of Mo facilitate the generation of superoxide anions and 1O2, respectively, facilitating photocatalytic reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


