利用 TMT 标记和信号增强技术的灵敏度增强型质谱分析策略,对生物制药中的宿主细胞蛋白质进行全面分析
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
背景宿主细胞蛋白(HCPs)是生物制药生产过程中宿主细胞中表达的杂质,可能会影响产品质量,并可能导致免疫原性反应或其他不良反应。基于质谱法(MS)的策略越来越被认为是一种有前途的 HCPs 分析方法,因为它能够在一次检测中同时定量数千种蛋白质。然而,考虑到系统中存在大量过剩的生物制药产品蛋白质,以及 HCPs 的丰度极低,HCPs 分析迫切需要灵敏的 MS 方法。结果在这项工作中,我们引入了一种新方法,利用宿主细胞裂解液作为增强通道,使用等位 TMT 标记法增强生物制药产品中残留 HCPs 的 MS 信号,从而将低丰度 HCPs 提高到当前 MS 可检测和可量化的水平,而无需使用富集或耗竭方法来避免 HCPs 原始浓度受到干扰。在 RM8671 NIST 单克隆抗体(mAb)的 HCPs 分析中,与现有记录(848 个蛋白质的 4541 个唯一肽)相比,我们的方法识别出了更多的 HCPs(3475 个蛋白质的 23844 个唯一肽),超越了之前的基准,显示了无与伦比的灵敏度和稳健性。此外,我们的工作流程成功鉴定了单克隆抗体 (mAb) 中浓度范围为 0.32 至 4.15ppm 的 48 个 UPS1 蛋白中的 44 个,证明了其在生物制药中深入分析 HCPs 的有效性。我们的质谱信号增强方法是 HCP 分析的一大进步,它将提高灵敏度、扩大 HCP 的范围与简化、稳健的工作流程相结合。该方法可在不干扰原始 HCPs 浓度的情况下,将 HCPs 定量在百万分之 1 的水平,这在该领域仍属罕见。本文章由计算机程序翻译,如有差异,请以英文原文为准。
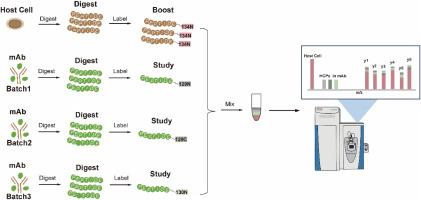
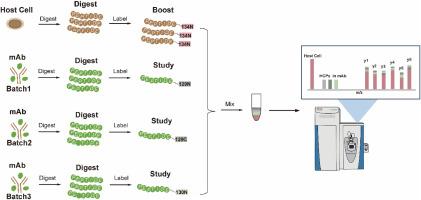
Comprehensive host cell proteins profiling in biopharmaceuticals by a sensitivity enhanced mass spectrometry strategy using TMT-labeling and signal boosting
Background
Host Cell Proteins (HCPs) are impurities expressed in host cells during the biopharmaceutical production process, whichmay compromise product quality and potentially leading to immunogenic reactions or other adverse effects. Mass spectrometry (MS)-based strategy is more and more considered as a promising method for HCPs analysis, since it is capable of simultaneously quantifying thousands of proteins in a single test. However, considering the large excess biopharmaceutical product protein present in the system and the extremely low abundance of HCPs, sensitive MS methods are urgently needed in HCPs analysis.
Results
In this work, we introduced a novel approach that leveraged host cell lysate as a boosting channel to enhance the MS signal of the residue HCPs in biopharmaceutical products using isobaric TMT labeling, thereby elevating the low-abundant HCPs to detectable and quantifiable levels of current MS without using enrichment or depletion method to avoid disturbance of the original concentration of the HCPs. Our method surpassed previous benchmarks by identifying a significantly higher number (23844 unique peptides for 3475 proteins) compared to existing records (4541 unique peptides for 848 proteins) for HCPs analysis in RM8671 NIST monoclonal antibody (mAb), demonstrating unparalleled sensitivity and robustness. Furthermore, our workflow successfully identified 44 of 48 UPS1 proteins across a concentration range of 0.32–4.15 ppm in monoclonal antibodies (mAbs), proving its effectiveness for in-depth HCPs analysis in biopharmaceuticals.
Significance
Present even at sub-ppm levels, HCPs may compromise the stability and safety of product proteins and alter pharmacokinetics or neutralization of therapeutic effects. Our MS signal enhancing method presented an advancement in HCP analysis, combining improved sensitivity and increased scale of HCPs with a streamlined and robust workflow. This method allowed HCPs quantification at <1 ppm level without disturbance of the original HCPs concentration, which is still rare in the field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


