从锂离子电池中金属集流体的选择看纳米结构铁氟化物的电化学合成与碳掺杂
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
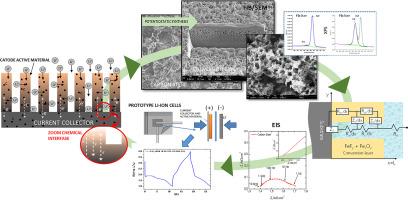
与传统的电极材料相比,依靠转化反应的材料具有增强容量的潜力,因此在锂离子电池中使用这种材料作为电极的研究得到了广泛的关注。就比容量而言,氟化铁尤其是一种有前途的替代材料。然而,这些材料往往面临着固有电导率低的挑战。文献中通常通过在活性材料中掺入碳颗粒并减小活性材料的粒径来解决这一问题。本研究探讨了在合成过程中将基底中的导电碳直接整合到氟化基活性材料中的可行性。合成采用一种简单的阳极法,直接在所选金属基底上进行,然后将其作为器件中的电流收集器。这种方法简化了合成过程,缩短了加工时间,而且无需在传统的活性材料-集流器界面上使用添加剂和粘合剂。在碳含量不同的钢基板上电化学合成了两层 FeF3。这些层被评估为可充电锂离子电池的阴极活性材料。利用电化学阻抗谱(EIS)和基于导电多孔电极的模型评估了碳对转换层电导率的影响。转换层的形态和厚度分析表明,孔隙大小和转换层厚度的增加与金属基底中的碳含量密切相关。在含碳量较高的基底上观察到的层具有最佳性能。使用电化学阻抗光谱和袋式电池中的电静电测试进一步评估了活性材料的电化学性能。与在纯铁上形成的转化层相比,碳钢转化层的电阻率降低,比电容和循环能力增强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrochemical Synthesis and Carbon Doping of Nanostructured Iron Fluorides from the Selection of Metal Current Collectors in Lithium-Ion Batteries
The use of materials that rely on conversion reactions as electrodes in lithium-ion batteries is extensively investigated due to their potential for enhanced capacity compared to traditional electrode materials. Iron fluorides, in particular, present a promising alternative in terms of specific capacity. However, these materials often face challenges related to low intrinsic conductivity. This issue is typically addressed in the literature by doping the active material with carbon particles and reducing the particle size of the active material. This study explores the feasibility of directly integrating conductive carbon from the substrate into the fluoride-based active material during the synthesis process. The synthesis employs a simple anodic method conducted directly on the chosen metallic substrate, which then functions as the current collector in the devices. This approach simplifies the synthesis process, reduces processing time, and eliminates the need for additives and binders at the conventional active material-current collector interface. Two FeF3 layers were electrochemically synthesized on steel substrates with different carbon contents. These layers were evaluated as cathode-active materials for rechargeable lithium-ion batteries. The influence of carbon on the conductivity of the conversion layer was assessed using Electrochemical Impedance Spectroscopy (EIS) with a model based on conductive porous electrodes. Morphological and thickness analyses of the layers showed a strong correlation between increased pore size and layer thickness and the carbon content in the metallic substrate. The optimal performance was observed with the layer on the substrate with higher carbon content. The electrochemical performance of the active material was further evaluated using electrochemical impedance spectroscopy and galvanostatic tests in pouch cells. The conversion layers derived from carbon steel exhibited reduced resistivity and enhanced specific capacitance and cyclability compared to layers formed on pure iron.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


