揭秘作为超级电容器电极材料的苹果酸锰
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
超级电容器因其快速的电荷存储/释放能力和较长的循环寿命而颇具吸引力。然而,其能量密度低于电池。研究界正在不断开发具有高能量和高功率能力的新型电极材料。有鉴于此,苹果酸锰作为超级电容器应用的电极材料应运而生。苹果酸锰是在不同的电池电压(2/3/4 V)下,通过时变测量法电沉积在不锈钢箔(面积:2 cm2)上的。X 射线衍射和红外光谱研究证实了苹果酸锰的形成,而电子显微镜研究则显示苹果酸锰沉积物的形态为纳米颗粒状。在 2 V 电压下电解沉积的苹果酸锰(MnM2)在 1 A g-1 时的比电容为 186 F g-1,在 0.1 M Mg(ClO4)2 中可稳定循环 10000 次。本文章由计算机程序翻译,如有差异,请以英文原文为准。
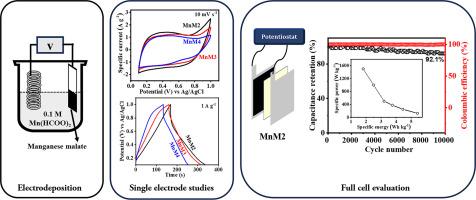
Unveiling Manganese Malate as an Electrode Material for Supercapacitors
Supercapacitors are attractive due to their rapid charge storage/release capabilities and long cycle-life. However, their energy density is lower than batteries. The research community is continuously developing new electrode materials with high energy and high power capabilities. In view of this, manganese malate is unveiled as an electrode material for supercapacitor applications. Manganese malate is electrodeposited on a stainless-steel foil (area: 2 cm2) by chronoamperometry at various cell voltages (2/3/4 V). While X-ray diffraction and infrared spectroscopic studies confirm the formation of manganese malate, electron microscopic studies reveal that the morphology of manganese malate deposit is nanoparticulate. Manganese malate electrodeposited at 2 V (MnM2) delivers a specific capacitance of 186 F g-1 at 1 A g-1, and stable cycle-life over 10000 cycles in 0.1 M Mg(ClO4)2. In addition, a symmetric supercapacitor fabricated using MnM2 delivers a specific energy of 6.25 Wh kg-1 at a specific power of 250.2 W kg-1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


