高度改性的富稀土硼酸盐玻璃中碎裂的 BO4 单元的存活率
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
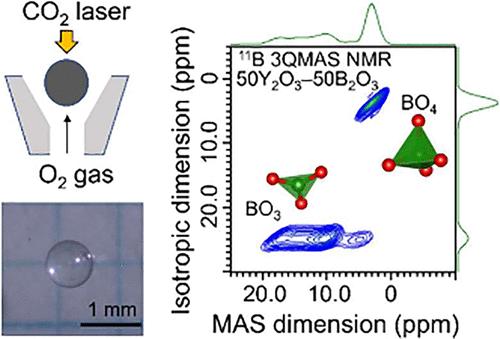
利用悬浮技术制备了高度改性的 La2O3-Y2O3-B2O3 三元玻璃。研究了 (50 - x)La2O3-xY2O3-50B2O3 玻璃和 (60 - y)La2O3-yY2O3-40B2O3 玻璃的热性能和结构特性。拉曼散射光谱显示,B 原子主要形成孤立的平面 BO3 三角形,与结晶 LaBO3 相似。这一过程与 La2O3 和 Y2O3 的比例无关。11B 魔角旋转核磁共振波谱证实,在成分高度改良的玻璃中,本应消失的 BO4 单元仍以碎片形式存在。在 50La2O3-50B2O3 玻璃中,约有 4% 的 B 原子形成了 BO4。这一比例随着 Y2O3 含量的增加而增加,在 50Y2O3-50B2O3 玻璃中达到最大值(15%)。利用 LaBO3 和 YBO3 晶体的 ab initio 计算对电子密度分布进行了比较,结果表明,Y-O 键原子核附近的电子比 La-O 键原子核附近的电子多。利用 LaBO3 和 YBO3 晶体的 ab initio 计算对电子密度分布进行了比较,结果表明,与 La-O 键中的电子相比,Y-O 键中的电子定位在原子核附近。因此,BO4 在高度改性玻璃中的非常规存在归因于 Y3+ 水平的增加,这导致了电子在原子核附近的定位。因此,高度改性玻璃中 BO4 和 BO3 的比例可以通过调整玻璃中改性稀土氧化物的含量来控制,这为玻璃科学打开了一扇新的大门。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Survival of Fragmented BO4 Units in Highly Modified Rare-Earth-Rich Borate Glasses
Highly modified La2O3–Y2O3–B2O3 ternary glasses were fabricated by using a levitation technique. The thermal and structural properties of (50 – x)La2O3–xY2O3–50B2O3 glasses and (60 – y)La2O3–yY2O3–40B2O3 glasses were investigated. Raman scattering spectra indicated that B atoms mainly formed isolated planar BO3 triangles similar to those of crystalline LaBO3. This process was independent of the ratio of La2O3 and Y2O3. 11B magic angle spinning nuclear magnetic resonance spectra confirmed that the BO4 units that should have disappeared in the glass with highly modified compositions remained as fragmented species. Approximately 4% of the B atoms formed BO4 in the 50La2O3–50B2O3 glass. This ratio increased with an increase in the Y2O3 content, and it reached its maximum value (15%) in the 50Y2O3–50B2O3 glass. Comparison of the electron density distribution was conducted using ab initio calculations of the LaBO3 and YBO3 crystals and indicated that more electrons localize near the atomic nuclei in the Y–O bond than in the La–O bonds. Comparison of the electron density distribution was conducted using ab initio calculations of the LaBO3 and YBO3 crystals and indicated that electrons in the Y–O bond localize near the atomic nuclei compared to those in the La–O bonds. Thus, the unconventional existence of BO4 in a highly modified glass is attributed to the increase in the level of Y3+, which causes the localization of electrons near the atomic nuclei. Thus, the ratio of BO4 and BO3 in highly modified glass can be controlled by tuning the glass content of modifier rare-earth oxides, which opens a new door to glass science.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


