硫醇和硒醇还原亚硝酸铁(II)-亚硝基化合物,通过{FeNO}7中间体生成二亚硝基铁络合物
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
Fe(II) 复合物 [Fe(6-COO--tpa)]1+ (1) 与 PhE- 和 NO2- 反应,分别生成 [Fe(6-COO--tpa)(EPh)] (E = S, 2a. Se, 3) 和 [Fe(6-COO--tpa)(κ2-O,O′-NO2)] (4) (6-COylOHm) ;Se, 3)和[Fe(6-COO--tpa)(κ2-O,O′-NO2)](4)(6-COOH-tpa 是双(2-吡啶基甲基)(6-羧基-2-吡啶基甲基)胺)。用 2 等量的 PhEH(E = S、Se)处理 4,可分别生成产率为 40%的 NO,以及 1 和 DNICs [Fe(EPh)2(NO)2]1-(E = S、Se)。用过量的 PhEH 处理 4 会以类似的产率产生 NO,而 4 则会转化为相同的 DNIC 和 2a/3(而不是 1)。有人认为 DNIC 是通过 PhE- 与原位生成的不稳定 {FeNO}7 中间体[Fe(6-COO--tpa)(NO)]1+(6)反应生成的。化合物 6 与 PhS- 反应生成 [Fe(SPh)2(NO)2]1-,从而支持了所提出的反应途径。最后,虽然两种独特的化合物具有内置质子源,即[Fe(6-COO--tpa)(S-C6H4-p-COOH)](7)和[Fe(6-COO--tpa)(S-C6H4-o-OH)](8),但它们与 0.4 与 HS-C6H4-p-COOH 和 HS-C6H4o-OH 处理后产生的 NO 收率要高得多(65-77%)。综合结果表明了 NO2- 的配位对质子辅助 NO2- 还原生成 NO 的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
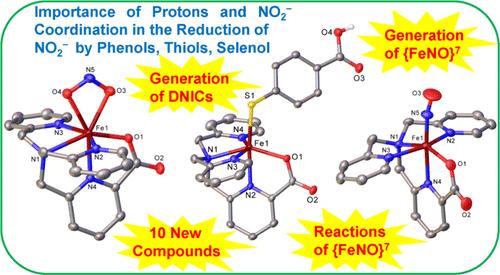
Reduction of Nitrite in an Iron(II)-Nitrito Compound by Thiols and Selenol Produces Dinitrosyl Iron Complexes via an {FeNO}7 Intermediate
Reaction of an Fe(II) complex, [Fe(6-COO–-tpa)]1+ (1), with PhE– and NO2– produced [Fe(6-COO–-tpa)(EPh)] (E = S, 2a; Se, 3) and [Fe(6-COO–-tpa)(κ2-O,O′-NO2)] (4), respectively (6-COOH-tpa is bis(2-pyridylmethyl)(6-carboxyl-2-pyridylmethyl)amine). Treatment of 4 with 2 equiv of PhEH (E = S, Se) produced NO in ∼40% yields, respectively, along with 1 and the DNICs, [Fe(EPh)2(NO)2]1– (E = S, Se). Treatment of 4 with excess PhEH produced NO in similar yields, while 4 was converted to the same DNICs and 2a/3 (instead of 1). The DNICs have been proposed to be generated via the reaction of PhE– with an in situ generated, unstable {FeNO}7 intermediate, [Fe(6-COO–-tpa)(NO)]1+ (6), which has also been synthesized separately. Compound 6 reacts with PhS– to generate [Fe(SPh)2(NO)2]1–, thus supporting the proposed reaction pathway. Finally, while the treatment of two unique compounds, featuring inbuilt proton sources, [Fe(6-COO–-tpa)(S-C6H4-p-COOH)] (7) and [Fe(6-COO–-tpa)(S-C6H4-o-OH)] (8), with 0.5 and 1 equiv of NO2– could produce NO only in 8–26% yields, treatment of 4 with HS-C6H4-p-COOH and HS-C6H4-o-OH produced NO in much higher yields (65–77%). The combined results delineated the importance of coordination of NO2– for the proton-assisted reduction of NO2– to generate NO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


