临床肿瘤药物开发中的生物标记物、生存和安全性综合建模
IF 15.2
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
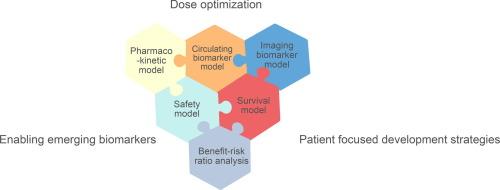
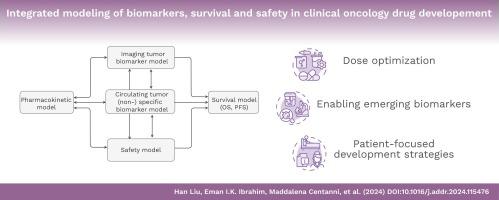
基于模型的方法,包括群体药代动力学-药效学模型,已成为肿瘤药物临床开发阶段的重要组成部分。在过去二十年中,模型已经发展到可以描述生物标志物和肿瘤大小的时间动态、治疗相关不良事件及其与生存的联系。综合模型在这里被定义为包含至少两个药效学/结果变量的模型,可用于通过模拟回答药物开发问题,例如支持探索亚组患者或其他肿瘤适应症的替代剂量策略和研究设计。随着监管机构进一步强调早期和个体化剂量优化以及以患者为中心的包容性开发战略,预计这些药物计量学方法将得到扩展。本综述概述了文献中的综合模型,举例说明了应用这些先进的药物计量学方法时需要考虑的因素,并展望了以模型为依据的抗癌药物开发有望进一步扩展的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Integrated modeling of biomarkers, survival and safety in clinical oncology drug development
Model-based approaches, including population pharmacokinetic-pharmacodynamic modeling, have become an essential component in the clinical phases of oncology drug development. Over the past two decades, models have evolved to describe the temporal dynamics of biomarkers and tumor size, treatment-related adverse events, and their links to survival. Integrated models, defined here as models that incorporate at least two pharmacodynamic/ outcome variables, are applied to answer drug development questions through simulations, e.g., to support the exploration of alternative dosing strategies and study designs in subgroups of patients or other tumor indications. It is expected that these pharmacometric approaches will be expanded as regulatory authorities place further emphasis on early and individualized dosage optimization and inclusive patient-focused development strategies. This review provides an overview of integrated models in the literature, examples of the considerations that need to be made when applying these advanced pharmacometric approaches, and an outlook on the expected further expansion of model-informed drug development of anticancer drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


