用于微调外泌体分泌以实现仿生学神经血管重塑的铁电活界面
IF 17.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
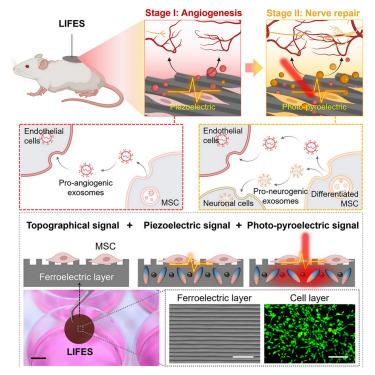
建立血管神经网络对组织再生至关重要。然而,现有的方法都无法复制不同细胞外线索在不同阶段依次发挥作用的生理过程,从而阻碍了神经血管的协同重塑。在这里,我们报告了一种用于微调外泌体分泌的铁电活界面(LIFES),它利用独特的地形和电信号(压电和光电),通过合理构建铁电层和活细胞层,持续产生生物活性外泌体。LIFES 具有模拟生理学的旁分泌效应,包括持续(∼192 h)、特定阶段的外泌体分泌、可调内容(∼8 倍的增长)和可编程的 microRNA(miRNA)载体(最初是促血管生成的,后来是促神经源的),克服了现有外泌体递送系统的局限性,如寿命短(∼24-48 h)、生物活性难以保存和载体不可改变。LIFES 可在体外和具有挑战性的糖尿病伤口模型中提高促进神经血管重塑的效果,为下一代智能材料和生物医学设备开辟了新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。

A ferroelectric living interface for fine-tuned exosome secretion toward physiology-mimetic neurovascular remodeling
Establishing vascular neural networks is critical for tissue regeneration. However, none of the existing approaches can replicate the physiological processes that varying extracellular cues sequentially play parts in different phases, thus hindering synergistic neurovascular remodeling. Here, we report a ferroelectric living interface for fine-tuned exosome secretion (LIFES) that harnesses unique topographical and electric (piezoelectric and photopyroelectric) signals and sustained generation of bioactive exosomes by rationally constructing a ferroelectric layer and a living cell layer. The LIFES exhibits physiology-mimicking paracrine effects, including sustained (∼192 h), phase-specific exosome secretion with tunable contents (∼8-fold increases) and programmable microRNA (miRNA) cargoes (initially pro-angiogenic and later pro-neurogenic), which overcome the limitations of the existing exosome delivery systems, such as short lifetime (∼24–48 h), difficult-to-preserve bioactivity, and non-changeable cargoes. LIFES allows for enhanced effectiveness in promoting neurovascular remodeling both in vitro and in challenging diabetic wound models, opening new avenues for next-generation intelligent materials and biomedical devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Matter
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
26.30
自引率
2.60%
发文量
367
期刊介绍:
Matter, a monthly journal affiliated with Cell, spans the broad field of materials science from nano to macro levels,covering fundamentals to applications. Embracing groundbreaking technologies,it includes full-length research articles,reviews, perspectives,previews, opinions, personnel stories, and general editorial content.
Matter aims to be the primary resource for researchers in academia and industry, inspiring the next generation of materials scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


