STAT3-STING-IFN 轴控制小细胞肺癌的转移扩散
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
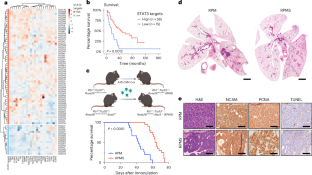
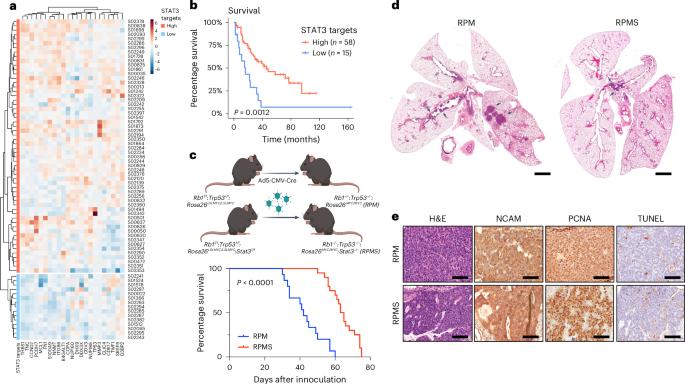
小细胞肺癌(SCLC)是一种侵袭性神经内分泌肿瘤,具有高转移潜力,总生存率约为 5%。包括小细胞肺癌在内的50%的肿瘤过度表达转录因子信号转导和激活因子3(STAT3),但它在小细胞肺癌的发展和转移中的作用尚不清楚。在这里,我们发现,STAT3 的缺失会限制原发性肿瘤的生长,但与之矛盾的是,它会通过促进免疫逃避来增强转移扩散。这是因为 STAT3 对维持免疫传感器干扰素(IFN)基因刺激器(STING)至关重要。如果没有 STAT3,环腺苷酸单磷酸鸟苷酸合成酶-STING 通路就会失去活性,导致 I 型干扰素分泌和 IFN 基因特征减少。重要的是,通过重新表达内源性 STING、加强 IFN 反应因子 7 的表达或服用重组 I 型 IFN 来恢复 IFN 信号转导,可以重建抗肿瘤免疫,抑制体内转移性 SCLC。这些数据显示了增强先天性免疫反应以阻止转移性 SCLC 的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A STAT3–STING–IFN axis controls the metastatic spread of small cell lung cancer
Small cell lung cancer (SCLC) is an aggressive neuroendocrine tumor characterized by a high metastatic potential with an overall survival rate of ~5%. The transcription factor signal transducer and activator of transcription 3 (STAT3) is overexpressed by >50% of tumors, including SCLC, but its role in SCLC development and metastasis is unclear. Here, we show that, while STAT3 deletion restricts primary tumor growth, it paradoxically enhances metastatic spread by promoting immune evasion. This occurs because STAT3 is crucial for maintaining the immune sensor stimulator of interferon (IFN) genes (STING). Without STAT3, the cyclic adenosine monophosphate–guanosine monophosphate synthase–STING pathway is inactive, resulting in decreased type I IFN secretion and an IFN gene signature. Importantly, restoration of IFN signaling through re-expression of endogenous STING, enforced expression of IFN response factor 7 or administration of recombinant type I IFN re-established antitumor immunity, inhibiting metastatic SCLC in vivo. These data show the potential of augmenting the innate immune response to block metastatic SCLC. Here, the authors show that signal transducer and activator of transcription 3 (STAT3) has dual functions in small cell lung cancer as its deletion inhibited primary tumor growth but boosted metastatic spread. These seemingly opposing functions are a result of the requirement of STAT3 for stimulator of interferon genes–interferon signaling in metastasis and the authors show how it can be targeted in mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


