祖先细胞身份网络的汰换实现了 C4 光合作用
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
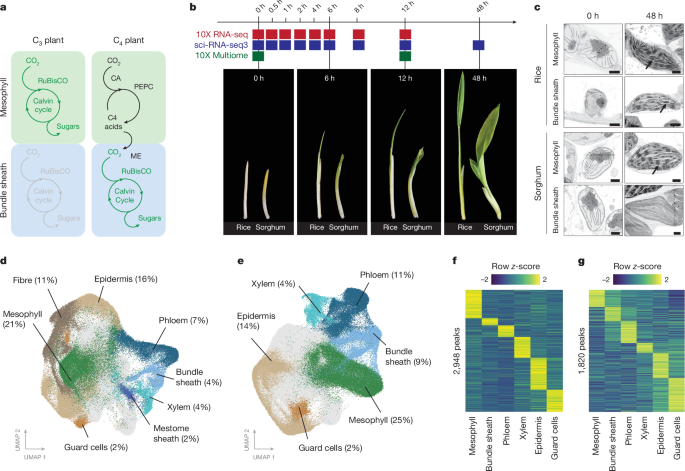
地球上产量最高的植物都采用 C4 光合作用,与祖先的 C3 途径相比,其效率提高了 50%1。在 60 多个 C4 品系中,二氧化碳的固定在组织间被分隔开来,束鞘细胞被光合作用激活2。目前还不清楚束鞘是如何获得这种高效光合作用的替代特性的。在这里,我们发现 C4 叶片中束鞘基因表达的变化与 C3 叶片中预先存在的顺式代码的获得有关。通过单核基因表达和染色质可及性图谱,我们发现了在主要作物 C3 水稻和 C4 高粱中定义束鞘特征的 DNA 结合单指(DOF)结构。在 C4 高粱束鞘细胞中强烈表达的光合作用基因获得了被 DOF 识别的顺式元件。我们的研究结果符合一个简单的模型,在该模型中,C4光合作用基于与束鞘特征相关的祖先顺式编码的招募。这种元素的获得利用了 C3 和 C4 叶片中细胞类型之间转录因子的稳定模式,从而激活了束鞘中的光合作用。我们的发现为复杂的 C4 途径的进化提供了分子见解,也可能指导 C3 作物中 C4 光合作用的合理工程,以提高作物产量和抗逆性3,4。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Exaptation of ancestral cell-identity networks enables C4 photosynthesis
C4 photosynthesis is used by the most productive plants on the planet, and compared with the ancestral C3 pathway, it confers a 50% increase in efficiency1. In more than 60 C4 lineages, CO2 fixation is compartmentalized between tissues, and bundle-sheath cells become photosynthetically activated2. How the bundle sheath acquires this alternate identity that allows efficient photosynthesis is unclear. Here we show that changes to bundle-sheath gene expression in C4 leaves are associated with the gain of a pre-existing cis-code found in the C3 leaf. From single-nucleus gene-expression and chromatin-accessibility atlases, we uncover DNA binding with one finger (DOF) motifs that define bundle-sheath identity in the major crops C3 rice and C4 sorghum. Photosynthesis genes that are rewired to be strongly expressed in the bundle-sheath cells of C4 sorghum acquire cis-elements that are recognized by DOFs. Our findings are consistent with a simple model in which C4 photosynthesis is based on the recruitment of an ancestral cis-code associated with bundle-sheath identity. Gain of such elements harnessed a stable patterning of transcription factors between cell types that are found in both C3 and C4 leaves to activate photosynthesis in the bundle sheath. Our findings provide molecular insights into the evolution of the complex C4 pathway, and might also guide the rational engineering of C4 photosynthesis in C3 crops to improve crop productivity and resilience3,4. Single-nucleus RNA-sequencing and chromatin-accessibility analyses in rice (a C3 plant) and sorghum (a C4 plant) provide insight into how C4 photosynthesis evolved in bundle-sheath cells, revealing that the acquisition of ancestral cis-elements was key.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


