儿童单纯疱疹性脑炎的脑免疫遗传缺陷
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
单纯疱疹病毒 1(HSV-1)脑炎(HSE)是人类最常见的散发性病毒性脑炎。它危及生命,发病高峰期在儿童期,即原发感染期。患有 HSE 的儿童并不特别容易受到其他感染,包括脑部以外组织的 HSV-1 感染。约有 8-10%的儿童病例是由于 19 个基因的单基因先天性错误引起的,其中三分之二是隐性遗传,而且大多数都显示出不完全的临床渗透性。因此,儿童 HSE 可能是散发性的,但也可能是遗传性的,这就需要采用新的诊断和治疗方法。在本综述中,我们将研究 HSE 患者大脑中细胞内在抗病毒免疫的基本细胞和分子机制。这些机制包括已知的(如 TLR3 途径中的突变)和以前未知的(如 TMEFF1 限制因子)抗病毒途径,它们可能依赖于(如 IFNAR1)或独立于(如通过 RIPK3)I 型干扰素。它们在大脑皮层或脑干神经元中发挥作用,分别是前脑和脑干感染的基础。相反,最严重的先天性白细胞错误,包括完全缺乏骨髓和/或淋巴血细胞,并不是 HSE 的基础。因此,脑驻留神经元内在免疫力的先天缺陷是 HSE 的基础,它将宿主对 HSV-1 的天然防御从免疫系统的白细胞扩大到机体的其他细胞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
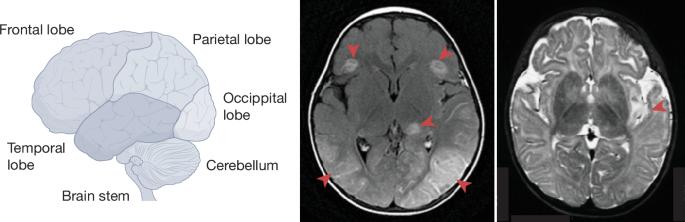
Genetic defects of brain immunity in childhood herpes simplex encephalitis
Herpes simplex virus 1 (HSV-1) encephalitis (HSE) is the most common sporadic viral encephalitis in humans. It is life-threatening and has a first peak of incidence in childhood, during primary infection. Children with HSE are not particularly prone to other infections, including HSV-1 infections of tissues other than the brain. About 8–10% of childhood cases are due to monogenic inborn errors of 19 genes, two-thirds of which are recessive, and most of which display incomplete clinical penetrance. Childhood HSE can therefore be sporadic but genetic, enabling new diagnostic and therapeutic approaches. In this Review, we examine essential cellular and molecular mechanisms of cell-intrinsic antiviral immunity in the brain that are disrupted in individuals with HSE. These mechanisms include both known (such as mutations in the TLR3 pathway) and previously unknown (such as the TMEFF1 restriction factor) antiviral pathways, which may be dependent (for example, IFNAR1) or independent (for example, through RIPK3) of type I interferons. They operate in cortical or brainstem neurons, and underlie forebrain and brainstem infections, respectively. Conversely, the most severe inborn errors of leukocytes, including a complete lack of myeloid and/or lymphoid blood cells, do not underlie HSE. Thus congenital defects in intrinsic immunity in brain-resident neurons that underlie HSE broaden natural host defences against HSV-1 from the leukocytes of the immune system to other cells in the organism. This article reviews evidence that has emerged over the past two decades indicating that herpes simplex encephalitis in children can result from monogenic defects of brain immunity to herpes simplex virus 1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


