阿尔茨海默氏症患者脑细胞外囊泡内的 Tau 细丝被拴住了
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
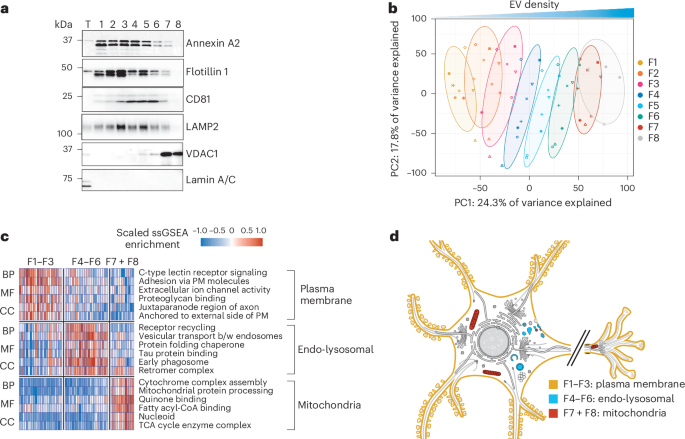
神经元中 tau 蛋白的异常组装是包括阿尔茨海默病(AD)在内的多种神经退行性疾病的病理标志。在阿尔茨海默病(AD)患者的中枢神经系统中,组装的tau蛋白与细胞外囊泡(EVs)结合,这与tau蛋白的清除和朊病毒样传播有关。然而,组装的tau种类和EV的身份以及它们是如何结合的尚不清楚。在这里,我们结合定量质谱法、低温电子断层扫描和单颗粒低温电子显微镜研究了AD患者的脑EVs。我们发现了主要由截短的 tau 组成的 tau 细丝,这些细丝被包裹在富含内溶酶体蛋白的 EVs 中。我们观察到多种丝状物相互作用,包括与将丝状物拴在EV限制膜上的分子的相互作用,这表明EV具有选择性包装功能。我们的发现将为研究EV介导的组装tau分泌的分子机制提供指导,并为靶向EV相关tau作为AD的潜在治疗和生物标记物策略提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Tau filaments are tethered within brain extracellular vesicles in Alzheimer’s disease
The abnormal assembly of tau protein in neurons is a pathological hallmark of multiple neurodegenerative diseases, including Alzheimer’s disease (AD). Assembled tau associates with extracellular vesicles (EVs) in the central nervous system of individuals with AD, which is linked to its clearance and prion-like propagation. However, the identities of the assembled tau species and EVs, as well as how they associate, are not known. Here, we combined quantitative mass spectrometry, cryo-electron tomography and single-particle cryo-electron microscopy to study brain EVs from individuals with AD. We found tau filaments composed mainly of truncated tau that were enclosed within EVs enriched in endo-lysosomal proteins. We observed multiple filament interactions, including with molecules that tethered filaments to the EV limiting membrane, suggesting selective packaging. Our findings will guide studies into the molecular mechanisms of EV-mediated secretion of assembled tau and inform the targeting of EV-associated tau as potential therapeutic and biomarker strategies for AD. Using cryo-electron tomography (cryo-ET) and proteomics, this study identifies the tethering of pathological tau filaments within defined brain extracellular vesicles in Alzheimer’s disease, shining light on the link between these vesicles and tau pathology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


