帕金森病药物恩他卡朋通过铁螯合作用破坏肠道微生物群平衡
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
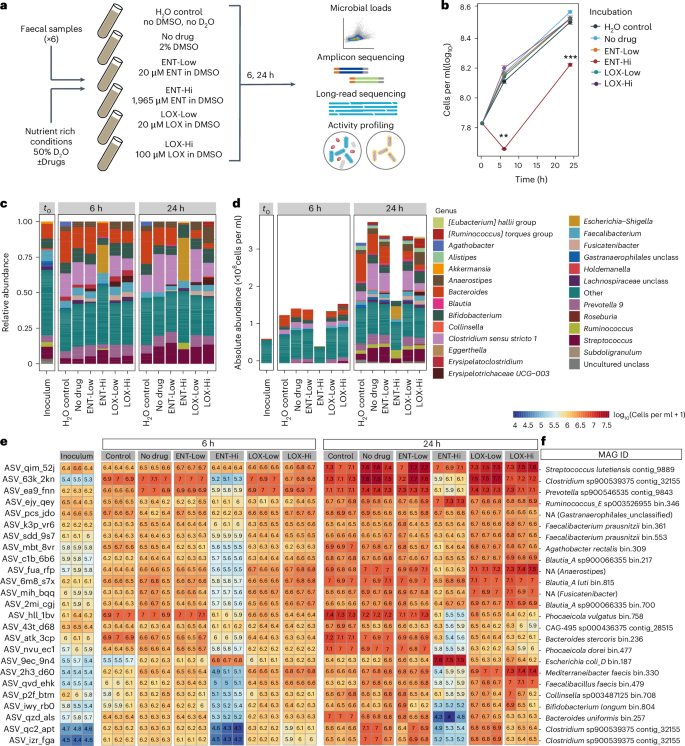
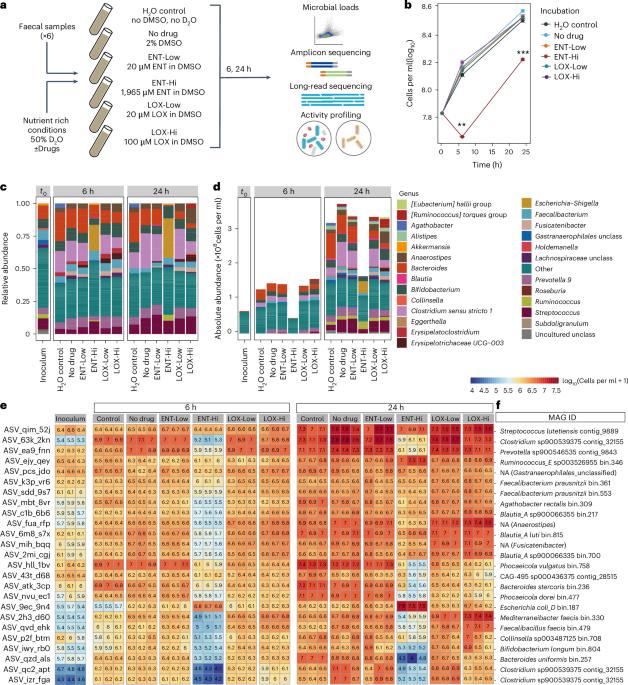
许多人类靶向药物会改变肠道微生物组,从而对宿主健康产生影响。然而,这些影响的机制尚不清楚。在这里,我们结合了定量微生物组图谱分析、长读元基因组学、稳定同位素探测和单细胞化学成像技术,研究了两种广泛使用的药物对肠道微生物组的影响。将生理相关浓度的治疗帕金森病的恩他卡朋或治疗精神分裂症的琥珀酸洛沙平与人体粪便样本进行体外培养。这两种药物对微生物活性的影响都很大,比对微生物数量的影响更大。从机理上讲,恩他卡朋能使可用铁元素复杂化并耗尽,从而导致肠道微生物组的组成和功能发生变化。通过补充微生物群可利用铁的水平,可以挽救微生物的生长。此外,恩他卡朋诱导的铁饥饿选择了编码抗微生物抗性和毒力基因的铁清除肠道微生物组成员。这些发现揭示了两种未得到充分研究的药物对整个微生物组的影响,并确定金属螯合是药物诱导微生物组紊乱的一种机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Parkinson’s disease drug entacapone disrupts gut microbiome homeostasis via iron sequestration
Many human-targeted drugs alter the gut microbiome, leading to implications for host health. However, the mechanisms underlying these effects are not well known. Here we combined quantitative microbiome profiling, long-read metagenomics, stable isotope probing and single-cell chemical imaging to investigate the impact of two widely prescribed drugs on the gut microbiome. Physiologically relevant concentrations of entacapone, a treatment for Parkinson’s disease, or loxapine succinate, used to treat schizophrenia, were incubated ex vivo with human faecal samples. Both drugs significantly impact microbial activity, more so than microbial abundance. Mechanistically, entacapone can complex and deplete available iron resulting in gut microbiome composition and function changes. Microbial growth can be rescued by replenishing levels of microbiota-accessible iron. Further, entacapone-induced iron starvation selected for iron-scavenging gut microbiome members encoding antimicrobial resistance and virulence genes. These findings reveal the impact of two under-investigated drugs on whole microbiomes and identify metal sequestration as a mechanism of drug-induced microbiome disturbance. Entacapone, a Parkinson’s disease medication, sequesters iron resulting in a selective inhibition of gut microbial activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


