通过简便的 Ti/Pd@MXene 滤波电极实现原子 H⁎ 介导的溴酸盐电化学还原
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
溴酸盐(BrO3-)是高级氧化水处理过程中常见的副产品。本研究合成了一种结合了 MXene 和 Pd 的催化剂,用于在直流模式下通过电化学还原消除 BrO3-。与 Ti/MXene 过滤器相比,所制备的 Ti/Pd@MXene 过滤器在还原 BrO3- 方面表现出更高的活性。在 pH 值为 5~7 和电流密度为 1.0~2.5 mA-cm-2 的条件下,Ti/Pd@MXene 过滤器去除 BrO3- 的性能令人满意。淬灭实验和 EPR 分析表明,原子氢(H⁎)介导的还原在 Ti/Pd@MXene 过滤器系统中占主导地位,对 84.2% 的 BrO3- 去除率做出了贡献,高于 Ti(7.1%)和 Ti/MXene (43.9%)。DFT 计算显示,在 MXene 上引入 Pd 纳米粒子降低了从 OH⁎-H⁎ 生成 H⁎ 的能垒,从而促进了 H⁎ 的形成。此外,Ti/Pd@MXene 过滤器具有良好的稳定性和适用性,在不同的水基质中可去除近 90% 的 BrO3-。此外,Ti/Pd@MXene 过滤器的能耗(0.348 kWh-mmol-1)比之前报道的更有竞争力,尤其是在高 BrO3- 去除率(≥75%)的情况下。这项工作凸显了一种有效的直流电催化过滤器,可诱导 H⁎ 介导的 BrO3- 电化学还原。本文章由计算机程序翻译,如有差异,请以英文原文为准。
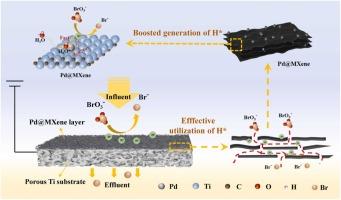
Atomic H*-mediated electrochemical reduction of bromate by a facile Ti/Pd@MXene filter electrode
Bromate (BrO3–) is a common by-product of advanced oxidation water treatment processes. In this study, a catalyst combining MXene and Pd was synthesized to eliminate BrO3– by electrochemical reduction in flow-through mode. The fabricated Ti/Pd@MXene filter showed superior activity for BrO3– reduction compared with Ti/MXene filter. A satisfactory BrO3− removal performance by Ti/Pd@MXene filter was obtained at pH values of 5–7 with a current density of 1.0–2.5 mA·cm–2. The mechanism explored by quenching experiments and EPR analysis demonstrated that atomic hydrogen (H*)-mediated reduction was dominant in the Ti/Pd@MXene filter system and contributed to 84.2 % of the BrO3− removal, which was greater than that of Ti (7.1 %) and Ti/MXene (43.9 %). DFT calculations revealed the introduction of Pd nanoparticles on MXene lowered the energy barrier for generating H* from OH* -H* , thus boosting H* formation. Furthermore, the Ti/Pd@MXene filter had favorable stability and applicability, and nearly 90 % of BrO3− could be eliminated in different water matrices. Moreover, energy consumption of the Ti/Pd@MXene filter was more competitive than that previously reported (0.348 kWh·mmol−1), especially for high BrO3− removal (≥75 %). This work highlighted an effective flow-through electrocatalytic filter to induce H* -mediated electrochemical reduction of BrO3−.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


