基于生物层干涉测量、多光谱分析和计算评估,揭示血清白蛋白与牛酪蛋白水解物中抗高血压肽 Val-Ala-Pro 的相互作用。
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-11-15
DOI:10.1016/j.saa.2024.125433
引用次数: 0
摘要
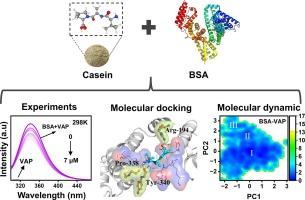
食物来源的血管紧张素转化酶抑制肽(ACEIP)对高血压有辅助治疗作用。研究人员以牛血清白蛋白(BSA)为模型转运蛋白,通过多光谱分析、生物层干涉仪(BLI)、等温滴定量热仪(ITC)、分子对接和分子动力学模拟等方法,探讨了BSA与酪蛋白水解的血管紧张素转化酶抑制肽Val-Ala-Pro(VAP)的相互作用机制。多光谱分析显示,VAP 与 BSA 形成的非共价复合物导致 BSA 的疏水性和 α 螺旋含量降低,从而揭示了 BSA 结构的解折。BLI 揭示了 BSA 与 VAP 的可逆结合过程。ITC 证实 VAP 与 BSA 的结合是一个自发过程,主要由熵驱动。分子对接和分子动力学模拟表明,VAP主要通过氢键、疏水相互作用、范德华力和静电力结合在BSA的位点II上。该研究为揭示 ACEIPs 的结构-活性关系提供了一种系统的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insight into the interaction of serum albumin with antihypertensive peptide Val-Ala-Pro from bovine casein hydrolysate based on the biolayer interferometry, multi-spectroscopic analysis and computational evaluation
Food-derived angiotensin-converting enzyme inhibitory peptide (ACEIP) has an effect in supportive therapeutic on hypertension. Bovine serum albumin (BSA) as a model transporter protein to explore the interaction mechanisms with casein-hydrolyzed ACEIP Val-Ala-Pro (VAP) by multi-spectroscopic, biolayer interferometry (BLI), isothermal titration calorimetry (ITC), molecular docking, and molecular dynamics simulations. Multi-spectroscopic analysis showed that the non-covalent complexes formed by VAP and BSA resulted in decreased hydrophobicity and α-helix contents on BSA, revealing the unfolding of the BSA structure. BLI revealed the reversible binding process of BSA to VAP. ITC confirmed that the combination of VAP to BSA was a spontaneous process mainly driven by entropy. Molecular docking and molecular dynamic simulations showed that VAP was primarily bound in site II of BSA by hydrogen bonding, hydrophobic interactions, van der Waals force, and electrostatic force. This study provides a systematic method to reveal the structure–activity relationship of ACEIPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


