人参皂苷化合物 K 通过双重抑制 GLS1 和 LDHA 抑制肝纤维化反应
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:肝纤维化是一个动态过程,其特点是肝星状细胞(HSCs)活化导致细胞外基质积累。人参皂苷化合物 K(CK)是人参皂苷母体的一种稀有衍生物,可显著改善代谢紊乱。目的:本研究旨在阐明 CK 对肝纤维化的保护作用,重点是代谢调节:方法:我们利用四氯化碳(CCl4)挑战、胆管结扎或蛋氨酸胆碱缺乏饮食建立了小鼠肝纤维化模型,并按指定剂量和时间间隔连续口服 CK。同时,我们还考察了 CK 对培养的造血干细胞代谢调节的影响,并研究了相关机制:结果:研究发现 CK 可减轻肝损伤,抑制小鼠模型的纤维化反应,并降低肝酶水平的升高。体外代谢组学分析强调了丙酮酸和谷氨酰胺代谢在代谢重塑中的关键作用。免疫组化染色显示,肝硬化患者乳酸脱氢酶 A (LDHA) (p = 0.014) 和谷氨酰胺酶 1 (GLS1) (p = 0.024) 的表达明显升高。在模型小鼠的肝脏和培养的造血干细胞中也发现了类似的变化。分子对接和生物层干涉测量法证明,CK 与 LDHA 和 GLS1 相互作用并抑制其活性。正如预期的那样,CK 可抑制糖酵解和谷氨酰胺酵解,减少依赖乳酸和α-酮戊二酸(α-KG)的造血干细胞生长。造血干细胞激活后,新陈代谢以 Myc 为关键调控因子进行重编程,转录控制 LDHA、GLS1 以及谷氨酰胺转运体 SLC1A5 和 SLC38A5。CK抑制了Myc的诱导,整合了糖酵解和谷氨酰胺酵解的调控,从而抵消了纤维化反应:结论:CK能抑制LDHA和GLS1的活性,从而抑制肝纤维化。这些发现为人参皂苷在保护肝脏,尤其是代谢紊乱方面的作用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
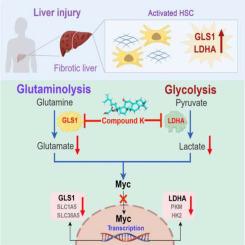
Ginsenoside compound K restrains hepatic fibrotic response by dual-inhibition of GLS1 and LDHA
Background
Liver fibrosis is a dynamic process marked by the accumulation of extracellular matrix due to hepatic stellate cells (HSCs) activation. Ginsenoside compound K (CK), a rare derivative of its parent ginsenosides, is known to significantly ameliorate metabolic disorders.
Purpose
The aim of this study was to elucidate the protective effects of CK against liver fibrosis with a focus on metabolic regulation.
Methods
We established liver fibrosis models in mice using carbon tetrachloride (CCl4) challenge, bile duct ligation, or a methionine-choline deficient diet, with continuous oral administration of CK at specified doses and intervals. Simultaneously, we examined the impact of CK on metabolic regulation in cultured HSCs and investigated the associated mechanisms.
Results
CK was found to alleviate liver injury and curb fibrotic responses in mouse models, as well as decrease elevated levels of liver enzyme. Metabolomic analysis in vitro highlighted the crucial roles of pyruvate and glutamine metabolism in metabolic remodeling. Immunohistochemical staining indicated significantly elevated expressions of lactate dehydrogenase A (LDHA) (p = 0.014) and glutaminase 1 (GLS1) (p = 0.024) in liver cirrhosis patients. Comparable alterations were noted in the liver of model mice and in cultured HSCs. Molecular docking and bio-layer interferometry demonstrated that CK interacts with and inhibits the activities of LDHA and GLS1. As expected, CK attenuated glycolysis and glutaminolysis, reducing HSC growth dependently on lactate and α-ketoglutarate (α-KG). Upon HSC activation, metabolism is reprogrammed with Myc as a key regulator, transcriptionally controlling LDHA, GLS1, and glutamine transporters SLC1A5 and SLC38A5. CK inhibited Myc induction, integrating glycolysis and glutaminolysis regulation to counteract the fibrotic response.
Conclusion
CK inhibited LDHA and GLS1 activities, thereby inhibiting hepatic fibrosis. These findings offer new insights into the role of ginsenosides in liver protection, especially regarding metabolic disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


