优化小儿急性淋巴细胞白血病维持阶段的地塞米松疗法:地塞米松和代谢物的群体药代动力学和药效学研究。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
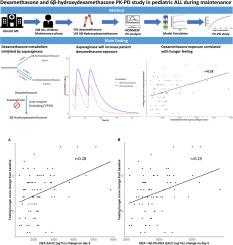
地塞米松对小儿急性淋巴细胞白血病(ALL)的治疗至关重要,但有关地塞米松及其代谢物药代动力学的研究却很少。我们的研究对地塞米松及其代谢物进行了全面的药代动力学-药效学分析,考察了它们与地塞米松引起的毒性之间的关系。研究收集了口服地塞米松(6 毫克/平方米/天)的小儿 ALL 患者在维持治疗阶段的峰值和谷值浓度。使用 NONMEM 研究了包括协变量在内的群体药代动力学。研究了药物及其活性代谢物暴露与不良反应之间的药代动力学(PK)和药效学(PD)相关性。研究收集了 104 名儿童(年龄在 3.0 -18.8 岁之间)的 382 份样本(地塞米松:191 份;6β-羟基地塞米松:191 份)。单室模型对数据进行了最佳描述。估计地塞米松的表观总清除率为 26 升/小时/70 千克,个体间变异率为 18%,表观分布容积为 123 升/70 千克,半衰期为 3.3 小时。协变量分析表明,同时服用天冬酰胺酶时,地塞米松的清除率和地塞米松代谢为 6β-hydroxydexamethasone 的程度均降低 50%。地塞米松药物暴露量与空腹饥饿评分之间存在统计学意义上的微弱正相关。通过抑制 CYP3A4 途径,地塞米松的暴露量在同时服用天冬酰胺酶时显著增加。我们的研究发现,地塞米松暴露量与饥饿感增加之间存在统计学意义上的显著但微弱的正相关。这些结果支持我们有必要开展更多研究,探讨如何在儿童 ALL 治疗中个性化地调整地塞米松剂量,并调整剂量以限制副作用,尤其是在联合用药的情况下。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Towards optimization of dexamethasone therapy in the maintenance phase of pediatric acute lymphoblastic leukemia: A population pharmacokinetic and pharmacodynamic study of dexamethasone and metabolite
Dexamethasone is crucial in pediatric acute lymphoblastic leukemia (ALL) treatment, however, studies regarding the pharmacokinetics of dexamethasone and its metabolites are scarce. Our study conducted a comprehensive pharmacokinetic-pharmacodynamic analysis of dexamethasone and metabolite, examining their association with dexamethasone-induced toxicity. Peak and trough concentrations were collected during the maintenance phase from pediatric ALL patients who received oral dexamethasone (6mg/m2/day). NONMEM was used to study the population pharmacokinetics including covariates. Pharmacokinetic (PK) and pharmacodynamic (PD) correlations between drug and its active metabolite exposure and adverse effects were examined. 382 samples (dexamethasone: n = 191; 6β-hydroxydexamethasone: n = 191) from 104 children (age range 3.0 -18.8 years) were collected. A one-compartment model described the data best. The estimated apparent dexamethasone total clearance was 26 L/h/70 kg with 18 % inter-individual variability, and an apparent volume of distribution of 123 L/70 kg, yielding a half-life of 3.3 h. Covariate analysis demonstrated that when asparaginase was co-administered, there was a 50 % reduction in both the clearance of dexamethasone and the extent to which dexamethasone was metabolized to 6β-hydroxydexamethasone. A statistically significant but weak positive correlation was observed between dexamethasone drug exposure and fasting hunger scores. Dexamethasone exposure significantly increased with asparaginase co-administration by inhibition of the CYP3A4 pathway. Our study found a statistically significant but weak positive correlation between dexamethasone exposure and increased hunger. These results support the need for more studies on how to personalize dexamethasone dosing in pediatric ALL treatment and adjust doses to limit side effects, especially in case of co-medication.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


