天然绿泥石中缓慢释放的铁(II)推动了以过硫酸盐为基础的持久高效地下水修复工程
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
过氧化二硫酸盐(PDS)的快速消耗以及过量铁元素对活性物质的竞争性失活限制了铁基催化剂活化 PDS 进行原位化学氧化(ISCO)的氧化持续时间和性能。在这里,天然绿泥石(富含铁的粘土矿物之一)被用作活化剂,以提高基于 PDS 的 ISCO 的性能和效率。研究发现,受污染地下水的酸化会促使水体中的铁(II)从绿泥石中缓慢释放,并成为多种活性物质的来源,包括羟基自由基 (-OH)、硫酸根自由基 (SO4--) 和铁(IV)。与 Fe(II)/PDS 和 ZVI/PDS 相比,亚氯酸盐/PDS 的氧化剂利用效率提高了 24-95%,氧化剂成本降低了 50%以上。长期实验表明,PDS 的持久性较强,被亚氯酸盐消耗的速度较慢,因此其氧化治污能力可保持一个月以上。这项工作不仅为地下水修复提出了一种有效、低成本且前景广阔的替代工艺,还证明了缓慢释放的 Fe(II) 在打破 ISCO 中过氧化物活化率与过氧化物利用效率之间的平衡方面具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
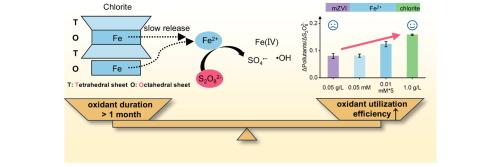
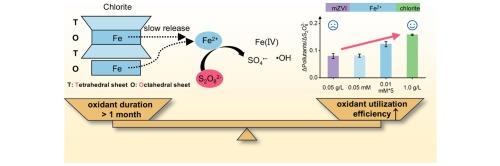
Long-lasting and efficient peroxydisulfate-based groundwater remediation driven by the slowly released Fe(II) from natural chlorite
The rapid depletion of peroxydisulfate (PDS) and the competitive inactivation of reactive species by excessive Fe restrict the oxidation duration and performance of iron (Fe)-based catalysts activated PDS for in situ chemical oxidation (ISCO). Here, natural chlorite, one of the Fe-rich clay minerals, is used as activator to enhance the performance and efficiency of PDS-based ISCO. It was found that acidification of contaminated groundwater drives the slowly release of aqueous Fe(II) from chlorite and serving as the source of multiple reactive species including hydroxyl radical (•OH), sulfate radical (SO4•−), and Fe(IV). Benefitting from the controlled release of Fe(II), the scavenging of oxidative species by Fe(II) is notably alleviated, leading to the oxidant utilization efficiency of chlorite/PDS improved by 24–95 % compared to the Fe(II)/PDS and ZVI/PDS, and the costs of oxidants reduced by over 50 %. Long-term experiments indicate that PDS is relatively persistent and slowly consumed by chlorite, hence the oxidative ability for pollution control remains for over one month. This work not only proposes an effective, low-cost and promising alternative process for groundwater remediation, but also demonstrates the significance of slowly released Fe(II) in breaking the trade-off between peroxide activation rate and peroxide utilization efficiency in ISCO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


