单细胞整合揭示炎症性肠道疾病中的变态反应
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
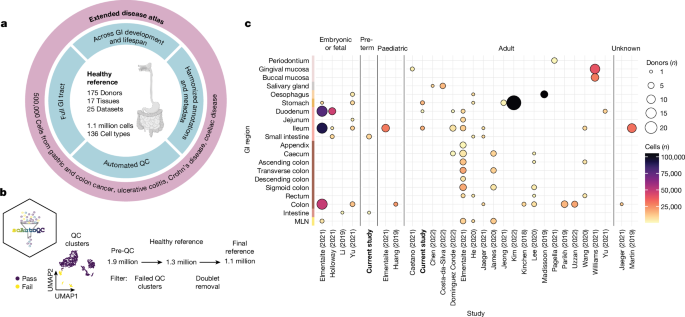
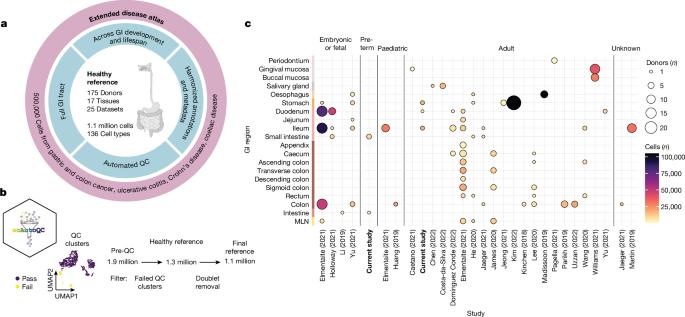
胃肠道是一个多器官系统,对有效摄取营养和屏障免疫至关重要。基因组学的进步和胃肠道疾病的激增1,2 推动了对健康和疾病中胃肠道组织细胞的编目工作3。在这里,我们系统整合了 25 个单细胞 RNA 测序数据集,涵盖了整个健康胃肠道的发育和成年期。我们使用新开发的自动质量控制方法(scAutoQC)对来自 189 个健康对照组的 385 个样本进行了统一处理,从而得到了一个包含约 110 万个细胞和 136 种细粒度细胞状态的健康参考图集。我们将胃肠道癌症、腹腔疾病、溃疡性结肠炎和克罗恩病等 12 种胃肠道疾病数据集锚定到该参考图集中。利用这一 160 万个细胞资源(gutcellatlas.org),我们发现肠道炎症疾病中的上皮细胞增生源自干细胞,与幽门腺和布鲁纳腺中的细胞具有转录相似性。虽然幽门腺上皮细胞变性以前与粘膜愈合有关4,但现在我们发现幽门腺上皮细胞变性通过招募免疫细胞(包括T细胞和中性粒细胞)与炎症有关。总之,我们描述了炎症诱导的干细胞变化,这种变化改变了粘膜组织结构并促进炎症进一步发展,这一概念适用于其他组织和疾病。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Single-cell integration reveals metaplasia in inflammatory gut diseases
The gastrointestinal tract is a multi-organ system crucial for efficient nutrient uptake and barrier immunity. Advances in genomics and a surge in gastrointestinal diseases1,2 has fuelled efforts to catalogue cells constituting gastrointestinal tissues in health and disease3. Here we present systematic integration of 25 single-cell RNA sequencing datasets spanning the entire healthy gastrointestinal tract in development and in adulthood. We uniformly processed 385 samples from 189 healthy controls using a newly developed automated quality control approach (scAutoQC), leading to a healthy reference atlas with approximately 1.1 million cells and 136 fine-grained cell states. We anchor 12 gastrointestinal disease datasets spanning gastrointestinal cancers, coeliac disease, ulcerative colitis and Crohn’s disease to this reference. Utilizing this 1.6 million cell resource (gutcellatlas.org), we discover epithelial cell metaplasia originating from stem cells in intestinal inflammatory diseases with transcriptional similarity to cells found in pyloric and Brunner’s glands. Although previously linked to mucosal healing4, we now implicate pyloric gland metaplastic cells in inflammation through recruitment of immune cells including T cells and neutrophils. Overall, we describe inflammation-induced changes in stem cells that alter mucosal tissue architecture and promote further inflammation, a concept applicable to other tissues and diseases. The study provides a comprehensive transcriptomic atlas of the human gastrointestinal tract across the lifespan, highlighting inflammation-induced changes in epithelial stem cells that alter mucosal architecture and promote further inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


