人类 HDAC6 感知缬氨酸丰度以调控 DNA 损伤
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
作为一种必需的支链氨基酸,缬氨酸对蛋白质合成、神经行为、造血和白血病的发展至关重要1,2,3。然而,细胞缬氨酸丰度对后续细胞功能的感知机制仍未确定。在这里,我们发现人类组蛋白去乙酰化酶 6(HDAC6)通过一个灵长类特异性 SE14 重复结构域直接结合缬氨酸,从而成为缬氨酸传感器。人(而非小鼠)HDAC6 在细胞核和细胞质中的穿梭受到细胞内缬氨酸水平的严格控制。缺乏缬氨酸会导致HDAC6滞留在细胞核中并诱导DNA损伤。从机理上讲,核定位的HDAC6与十-十一转位2(TET2)结合并使其去乙酰化,从而启动活跃的DNA去甲基化,通过胸腺嘧啶DNA糖基化酶驱动的切除促进DNA损伤。限制膳食中的缬氨酸可抑制异种移植和患者衍生异种移植模型中的肿瘤生长,并增强 PARP 抑制剂的疗效。总之,我们的研究确定了人类 HDAC6 是一种缬氨酸传感器,它能在缬氨酸被剥夺时介导活跃的 DNA 去甲基化和 DNA 损伤,并强调了限制膳食缬氨酸治疗癌症的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
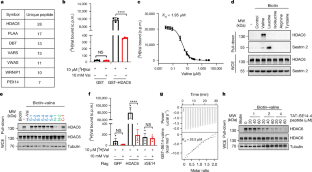

Human HDAC6 senses valine abundancy to regulate DNA damage
As an essential branched amino acid, valine is pivotal for protein synthesis, neurological behaviour, haematopoiesis and leukaemia progression1–3. However, the mechanism by which cellular valine abundancy is sensed for subsequent cellular functions remains undefined. Here we identify that human histone deacetylase 6 (HDAC6) serves as a valine sensor by directly binding valine through a primate-specific SE14 repeat domain. The nucleus and cytoplasm shuttling of human, but not mouse, HDAC6 is tightly controlled by the intracellular levels of valine. Valine deprivation leads to HDAC6 retention in the nucleus and induces DNA damage. Mechanistically, nuclear-localized HDAC6 binds and deacetylates ten-eleven translocation 2 (TET2) to initiate active DNA demethylation, which promotes DNA damage through thymine DNA glycosylase-driven excision. Dietary valine restriction inhibits tumour growth in xenograft and patient-derived xenograft models, and enhances the therapeutic efficacy of PARP inhibitors. Collectively, our study identifies human HDAC6 as a valine sensor that mediates active DNA demethylation and DNA damage in response to valine deprivation, and highlights the potential of dietary valine restriction for cancer treatment. The histone deacetylase HDAC6 is a sensor of valine levels, and the abundance of valine in cells regulates DNA damage and cell homeostasis functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


