探索仿生烯烃羟基化机制:当二铁金属中心遇到含硫配体时
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在温和的反应条件下高效、选择性地进行炔烃羟基化是化学转化中一项具有挑战性的任务。为了实现这一目标,我们中的一位最近报告了一种基于含硫配体 BCPOM 的高效铁催化剂的实验突破。然而,这种前景广阔的仿生物催化的确切机理仍然难以捉摸。在此,我们基于密度泛函理论建模并结合实验结果,成功揭示了这一仿生物炔羟化反应的意外机理。在二铁/BCPOM 的这一机理中,我们发现配体的二硫基通过同时形成一个 S-O 键来协助过氧物种的 O-O 裂解,从而产生了一个不常见的二价二铁-过氧中间体,作为随后炔烃羟基化反应的真正氧化剂。因此,所揭示的二铁/BCPOM羟基化机制不仅与单核血红素酶 P450 不同,而且与二铁非血红素酶 T4MO 也有很大区别。与 NIH 转化实验结果一致,该机制还能对一些以前未探索过的底物进行实验证实的区域选择性预测。含硫配体在通过形成 S-O 键协助 O-O 裂解方面发挥了意想不到的作用,这进一步拓展了我们对硫如何促进铁催化反应的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
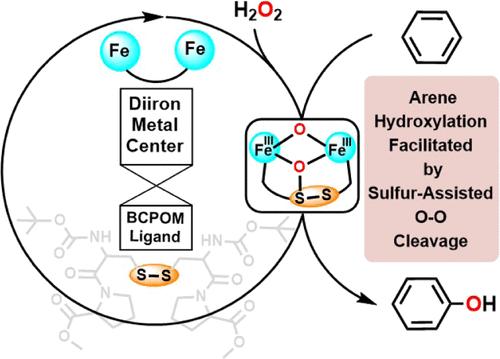
Exploring the Mechanism of Biomimetic Arene Hydroxylation: When a Diiron Metal Center Meets a Sulfur-Containing Ligand
Efficient and selective arene hydroxylation under mild reaction conditions is a challenging task in chemical transformation. To achieve this goal, one of us recently reported an experimental breakthrough of a highly efficient iron catalyst based on the sulfur-containing ligand BCPOM. However, the exact mechanism underlying this promising biomimetic catalysis remained elusive. Herein, based on density functional theory modelings combined with experimental results, we successfully revealed an unexpected mechanism of this biomimetic arene hydroxylation. In this mechanism of diiron/BCPOM, the disulfide group of the ligand was found to assist the O–O cleavage of the peroxo species by concomitantly forming an S–O bond, which thus generated an uncommon diferric diiron-oxo intermediate as the real oxidant for the subsequent arene hydroxylation. In this way, the revealed hydroxylation mechanism with diiron/BCPOM differs not only from the mononuclear heme enzyme P450 but also from the diiron nonheme enzyme T4MO substantially. Consistent with the NIH shift experimental results, this mechanism also enabled the experimentally confirmed regioselectivity prediction for some substrates unexplored previously. The unexpected role played by the sulfur-containing ligand in assisting the O–O cleavage by forming the S–O bond further expands our knowledge on how sulfur can facilitate the iron-catalyzed reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


