氧进化反应下铅电极上生成的电催化剂层的操作鉴定
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
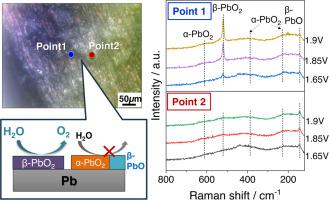
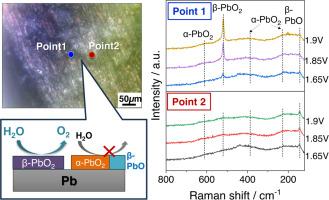
铅(Pb)基材料通常在各种电化学系统中用作电极。然而,作为电催化剂的表面薄膜在氧进化反应(OER)环境中的化学状态尚未明确。在本研究中,我们通过操作显微电化学(EC)拉曼光谱研究了在硫酸溶液中进行 OER 时铅电极上生成的表面薄膜的化学状态和微观结构。电化学拉曼光谱显示,表面薄膜的铅相关化合物和晶体结构取决于所施加的电位和分析点。值得注意的是,在主要分析点,检测到一条尖锐的β-PbO2 带,它是 OER 电位区的主相,其强度随施加电位的增加而增加。另一方面,在另一个分析点,β-PbO2 的条带没有出现在同一电位区域。结果表明,局部环境会显著影响铅电极上电解质生成的表面薄膜的化学状态,而 β-PbO2 是主要的 OER 活性位点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Operando identification of electrocatalyst layer generated on lead electrode under oxygen evolution reaction
Lead (Pb)-based materials are conventionally used as electrodes in various electrochemical systems. However, the chemical state of the surface films working as electrocatalysts under the oxygen evoltuion reaction (OER) has not been clarified. In this study, we investigated the chemical state and microstructure of surface films generated on a Pb electrode during OER in a sulfuric acid solution by operando microscopic electrochemical (EC) Raman spectroscopy. The EC-Raman spectra revealed that the Pb-related compounds and crystal structure of the surface films depend on the applied potential and analysis point. Notably, at the main analysis point, a sharp band of β-PbO2 was detected as the main phase in the potential region for OER and its intensity increased with increase in the applied potential. On the other hand, at another analysis point, the band for β-PbO2 did not appear in the same potential region. Instead, α-PbO2 and β-PbO, which have a lower electronic conductivity than β-PbO2, were detected, and the band intensities were almost unchanged by increasing the potential. The results indicate that the local environment significantly affects the chemical states of EC-generated surface films on a Pb electrode and β-PbO2 acts as the main OER active site.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


