掺杂 N 的三维碳纳米片:简易合成和高浓度染料吸附
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
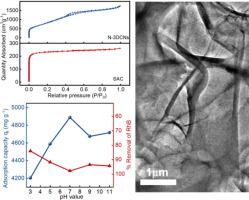
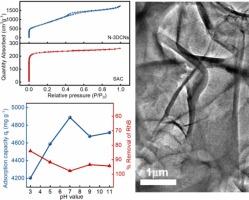
染料污染废水的净化一直是研究热点,但由于污染物浓度高、种类多,净化也是一项挑战。本研究结合煅烧和活化技术,以氮基三乙酸三钠盐为基础,设计了一种高效的氮掺杂三维碳纳米片(N-3DCNs)吸附剂,其比表面积为 2592.50 m2 g-1,平均孔体积为 2.68 m3/g。N-3DCNs 的微观结构和表面电位表明,随着溶液 pH 值从 3 升至 11,主材料晶格中大量的 N 杂原子可有效优化 Zeta 电位,使其从 1.53 降至 -31.08 mV。因此,所制备的 N-3DCNs 具有丰富的吸附位点和较强的静电作用,具备了高效、选择性吸附阳离子染料的必要条件。当阳离子罗丹明和阴离子甲基橙分别为 700 mg/L 的高浓度工业染料污染废水时,N-3DCNs 在 60 分钟内的去除率分别为 97.97 % 和 89.13 %。此外,在阳离子浓度为 1000 mg/L 时,N-3DCNs 的吸附容量对 pH 的耐受性较宽,在 pH = 7 时吸附容量最大为 4888.70 mg/g,在 pH = 3 时吸附容量最小为 4203.77 mg/g,衰减率仅为 14%。染料吸附的动力学机制可以用假二阶动力学模型很好地解释,表明存在化学吸附行为,同时与线性朗缪尔等温模型的拟合度较高。N-3DCNs 具有突破性的优异吸附性能主要归功于其高比表面积和带负电荷的活性位点,从而促进了协同吸附。本文章由计算机程序翻译,如有差异,请以英文原文为准。
N-doped three-dimensional carbon nanosheets: Facile synthesis and high-concentration dye adsorption
Purification of dye-contaminated wastewater has always been a research hotspot, yet also a challenge, due to high concentration and species diversity of pollutants. The present study designs an efficient nitrogen-doped 3D carbon nanosheets (N-3DCNs) adsorbent with 2592.50 m2 g−1 of specific surface area and 2.68 m3/g of average pore volume based on nitrilotriacetic acid trisodium salt by combining calcination and activation techniques. Microstructure and surface potential of N-3DCNs indicate that a large number of N heteroatoms in the lattice of main material can effectively optimize Zeta potential from 1.53 to −31.08 mV with solution pH increases from 3 to 11. So, as-prepared N-3DCNs possesses necessary conditions for efficient and selective adsorption of cationic dyes due to the abundant adsorption sites and strong electrostatic interactions. When 700 mg/L of cationic rhodamine and anionic methyl orange are respectively used as high concentration industrial dye-contaminated wastewater, N-3DCNs shows 97.97 % and 89.13 % of removal efficiency within 60 min. Furthermore, the adsorption capacity of N-3DCNs displays a wider pH tolerance at 1000 mg/L of cationic concentration, with a maximum adsorption capacity of 4888.70 mg/g at pH = 7 and a minimum value of 4203.77 mg/g at pH = 3, only 14 % of attenuation rate. The kinetics mechanism of dye adsorption could be well explained by pseudo-second-order kinetic model, suggesting chemisorption behavior, while fitting better with the linear Langmuir isothermal model. The groundbreaking and exceptional adsorption performances of N-3DCNs can be attributed primarily to the high specific surface area and negatively charged active sites, facilitating synergistic adsorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


