基于酰基烯酮-炔烃环加成法的立方酮的合成
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在此报告了一条全合成榄香烯的简明路线。形成炔酮中间体的关键策略涉及 Fe(III)/TEMPO 催化的 1,3-二氢异苯并呋喃分子的有氧氧化。通过这种醚氧化作用同时安装酮醛,可以有效地形成所需的炔酮中间体。酰基烯酮与炔酮的独特形式[4 + 2]环加成法证实了榄香烯的简洁全合成,该合成只需 7 个步骤即可完成,总收率为 9.4%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
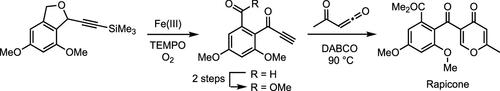
Synthesis of Rapicone Based on Acyl Ketene–Alkyne Cycloaddition
We report herein a concise route for the total synthesis of rapicone. The key strategy to form the ynone intermediate involves an Fe(III)/TEMPO-catalyzed aerobic oxidation of a 1,3-dihydroisobenzofuran moiety. This ether oxidation for the simultaneous installation of the keto aldehyde allowed the effective formation of the required ynone intermediate. The concise total synthesis of rapicone is substantiated by the unique formal [4 + 2] cycloaddition of acyl ketene with alkynone and is completed in 7 steps with 9.4% overall yield.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


