获得性两性霉素 B 抗性会导致适应性权衡,而这种权衡可通过念珠菌的补偿性进化来缓解
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
由于白色念珠菌对抗真菌药物,尤其是两性霉素 B(AMB)产生耐药性,30% 至 60% 的临床分离菌株对 AMB 产生耐药性,因此白色念珠菌日益受到关注。然而,人们对 AMB 的耐药性机制仍然知之甚少。在这里,我们研究了 441 个体外和体内进化的 C. auris 菌系,这些菌系来自 4 个对 AMB 敏感的不同支系的临床菌株。遗传和固醇分析显示,由于临床上罕见的固醇生物合成基因 ERG6、NCP1、ERG11、ERG3、HMG1、ERG10 和 ERG12 的变异,导致四种主要类型的固醇改变。此外,在抗药性进化过程中还出现了 4 号和 6 号染色体的非整倍体。适应性权衡表型分析和数学建模确定了多种依赖于菌株和机制的适应性权衡。CDC25 的变异可消除适应性权衡,从而提高感染能力。这可能是临床治疗中获得性 AMB 耐药性的原因之一。我们的研究结果突出表明,固醇调节机制和适应性权衡补偿是临床环境中 AMB 治疗失败的风险所在。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acquired amphotericin B resistance leads to fitness trade-offs that can be mitigated by compensatory evolution in Candida auris
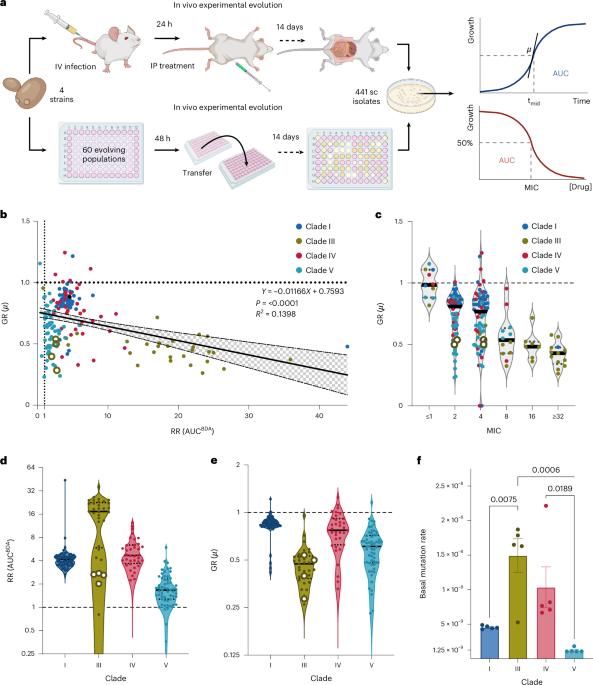
Candida auris is a growing concern due to its resistance to antifungal drugs, particularly amphotericin B (AMB), detected in 30 to 60% of clinical isolates. However, the mechanisms of AMB resistance remain poorly understood. Here we investigated 441 in vitro- and in vivo-evolved C. auris lineages from 4 AMB-susceptible clinical strains of different clades. Genetic and sterol analyses revealed four major types of sterol alterations as a result of clinically rare variations in sterol biosynthesis genes ERG6, NCP1, ERG11, ERG3, HMG1, ERG10 and ERG12. In addition, aneuploidies in chromosomes 4 and 6 emerged during resistance evolution. Fitness trade-off phenotyping and mathematical modelling identified diverse strain- and mechanism-dependent fitness trade-offs. Variation in CDC25 rescued fitness trade-offs, thereby increasing the infection capacity. This possibly contributed to therapy-induced acquired AMB resistance in the clinic. Our findings highlight sterol-modulating mechanisms and fitness trade-off compensation as risks for AMB treatment failure in clinical settings. The fungal pathogen Candida auris can acquire amphotericin B resistance through clinically rare mutations in sterol biosynthesis genes but at a certain fitness cost, which reduces its infection potential. Compensatory evolution can, however, mitigate this cost.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


