肠道细菌感染刺激胆汁代谢物重塑,促进肠道平衡
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
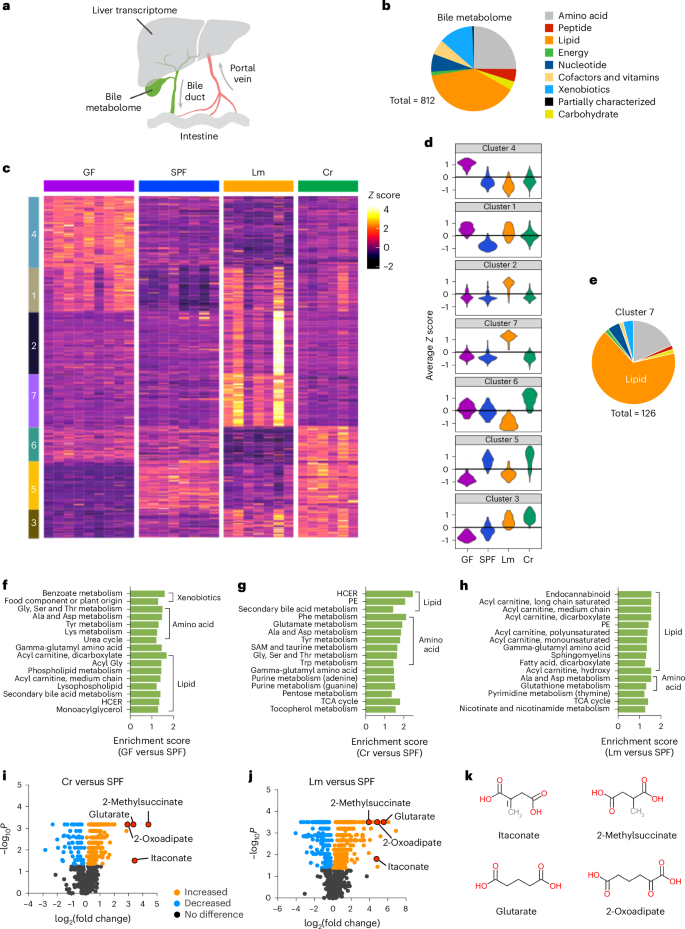
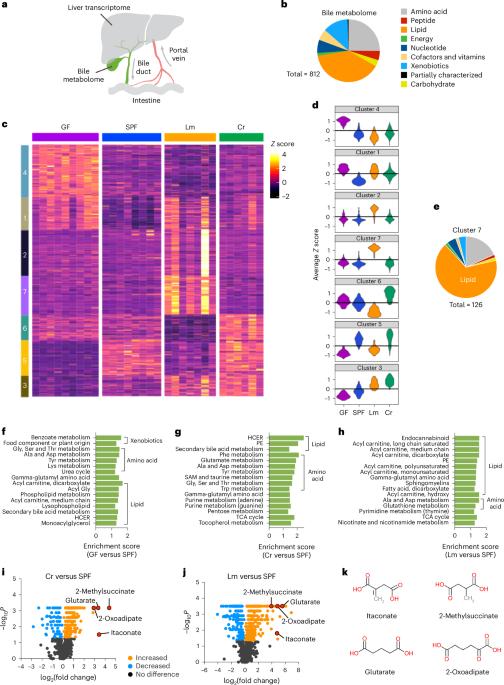
胆汁是一种对脂肪吸收至关重要的水溶液,由肝脏分泌到十二指肠。尽管对胆汁盐进行了广泛的研究,但胆汁中其他成分的特征还不太清楚。在这里,我们对来自无特定病原体、无细菌、受柠檬杆菌感染或受李斯特菌感染的小鼠的胆汁进行了全局代谢组学分析,并确定了由 812 种代谢物组成的代谢组,这些代谢物会因微生物群和肠道感染而改变。肝脏转录组学确定了肠道感染触发的通路,这些通路可能是胆汁重塑的基础。肠道感染增加了胆汁中四种二羧酸盐的水平,包括伊它康酸。对 Acod1-/- 小鼠的分析表明,伊它康酸的增加也会增加簇细胞的丰度,改变微生物群的组成和功能(通过元基因组分析检测到),并调节宿主防御,从而减少霍乱弧菌的定植。我们的数据表明,肠道感染相关信号在肠道和肝脏之间传递,并诱导形成胆汁代谢组的转录程序,从而改变胆汁的免疫调节和宿主防御功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enteric bacterial infection stimulates remodelling of bile metabolites to promote intestinal homeostasis
The liver makes bile, an aqueous solution critical for fat absorption, which is secreted into the duodenum. Despite extensive studies on bile salts, other components of bile are less well characterized. Here we used global metabolomic analysis on bile from specific-pathogen-free, germ-free, Citrobacter rodentium-infected or Listeria monocytogenes-infected mice and identified a metabolome of 812 metabolites that were altered by both microbiota and enteric infection. Hepatic transcriptomics identified enteric-infection-triggered pathways that probably underlie bile remodelling. Enteric infection increased levels of four dicarboxylates in bile, including itaconate. Analysis of Acod1−/− mice indicated that increased itaconate also increased tuft cell abundance, altered microbiota composition and function as detected by metagenomic analysis, and modulated host defence, leading to reduced Vibrio cholerae colonization. Our data suggest that enteric-infection-associated signals are relayed between the intestine and liver and induce transcriptional programmes that shape the bile metabolome, modifying the immunomodulatory and host defence functions of bile. Metabolomic analysis of bile after infection by enteric bacteria in mice reveals composition changes including increased itaconate levels that promote intestinal homeostasis and reduce Vibrio cholerae colonization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


