UBXN9 控制着 GLUT4 介导的 RIG-I 样受体和信号传导的空间限制
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
细胞质中的 RIG-I 样受体(RLR)能识别病毒 RNA 并启动先天性抗病毒免疫。RLR 信号还通过葡萄糖转运体(GLUT)触发糖酵解重编程,而葡萄糖转运体在抗病毒免疫中的作用尚不明确。在这里,我们揭示了胰岛素反应性 GLUT4 可抑制 RLR 信号,而与脂肪和肌肉组织中的葡萄糖摄取无关。在稳定状态下,GLUT4 被泛素调节 X 结构域 9(UBXN9,TUG)困在高尔基体基质中。RNA 病毒感染后,GLUT4 被释放并转运到细胞表面,在那里它与大量的细胞膜 RLR 进行空间隔离,阻止它们激活 IFN-β 反应。UBXN9 基因缺失会导致 GLUT4 构成性转运、RLRs 封存和抗病毒免疫功能减弱,而 GLUT4 基因缺失则会增强 RLR 信号转导。值得注意的是,GLUT4 表达的减少与以干扰素反应亢进为特征的人类炎症性肌病有着独特的联系。总之,我们的研究结果证明了一种非经典的 UBXN9-GLUT4 轴,它通过质膜拴系细胞膜 RLR 来控制抗病毒免疫。本文章由计算机程序翻译,如有差异,请以英文原文为准。
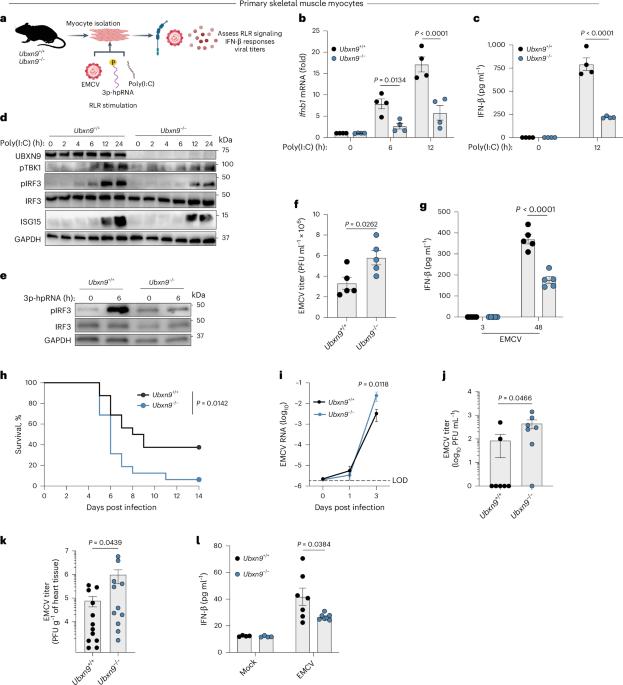
UBXN9 governs GLUT4-mediated spatial confinement of RIG-I-like receptors and signaling
The cytoplasmic RIG-I-like receptors (RLRs) recognize viral RNA and initiate innate antiviral immunity. RLR signaling also triggers glycolytic reprogramming through glucose transporters (GLUTs), whose role in antiviral immunity is elusive. Here, we unveil that insulin-responsive GLUT4 inhibits RLR signaling independently of glucose uptake in adipose and muscle tissues. At steady state, GLUT4 is trapped at the Golgi matrix by ubiquitin regulatory X domain 9 (UBXN9, TUG). Following RNA virus infection, GLUT4 is released and translocated to the cell surface where it spatially segregates a significant pool of cytosolic RLRs, preventing them from activating IFN-β responses. UBXN9 deletion prompts constitutive GLUT4 translocation, sequestration of RLRs and attenuation of antiviral immunity, whereas GLUT4 deletion heightens RLR signaling. Notably, reduced GLUT4 expression is uniquely associated with human inflammatory myopathies characterized by hyperactive interferon responses. Overall, our results demonstrate a noncanonical UBXN9-GLUT4 axis that controls antiviral immunity via plasma membrane tethering of cytosolic RLRs. Wang and colleagues show that in skeletal muscle cells and cardiomyocytes, the glucose transporter GLUT4 is a negative regulator of RIG-I-like receptor signaling during viral infection by redistributing RIG-I and MDA5 to the plasma membrane and attenuating interferon responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


