以 CRISPR-Cas9 和聚集诱导发光光敏剂为介导的先进纳米平台可促进癌症抗肿瘤治疗
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
免疫疗法与光疗相结合正在成为治疗全能癌症的一种前景广阔的策略。本研究将簇状规则间隔短回文重复序列(CRISPR)相关蛋白9(Cas9)系统、聚集诱导发射(AIE)光敏剂(PS)和聚乙烯亚胺/透明质酸表面涂层结合起来,构建了一种多功能纳米平台,称为TCPH纳米颗粒(NPs),用于癌症的综合治疗。TCPH NPs具有高效的活性氧(ROS)产生、良好的光热转换、程序性死亡配体1(PD-L1)消除能力和有效的细胞内运输等内在功能。产生的 ROS 和高热不仅能消除原发性肿瘤,还能调节肿瘤免疫微环境。PD-L1 基因组破坏明显增强了其疗效,尤其是在肿瘤转移和复发方面。此外,还开发了出色的多模态成像导航。在不同的肿瘤模型中证实了卓越的治疗性能,这意味着这种光热疗法和免疫疗法的协同策略为新兴的 CRISPR 介导的纳米药物提供了一种范式转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
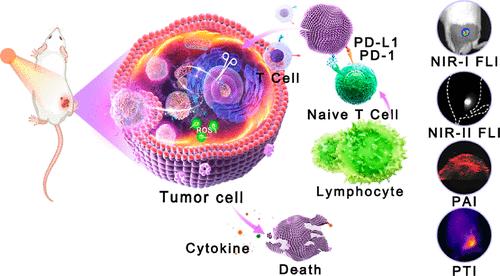
Advanced Nanoplatform Mediated by CRISPR-Cas9 and Aggregation-Induced Emission Photosensitizers to Boost Cancer Theranostics
Immunotherapy combined with phototherapy is emerging as a promising strategy to treat omnipotent cancers. In this study, a clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9) system, aggregation-induced emission (AIE) photosensitizer (PS) and surface coating of polyethylene imine/hyaluronic acid were combined to construct a multifunctional nanoplatform, denoted as TCPH nanoparticles (NPs), for comprehensive cancer theranostics. TCPH NPs are featured by intrinsic functions including efficient reactive oxygen species (ROS) production, good photothermal conversion, programmed death-ligand 1 (PD-L1)-eliminating capability, and effective intracellular transport. The generated ROS and hyperthermia do not only achieve primary tumor elimination but also regulate the tumor immune microenvironment. Genomic disruption of PD-L1 conspicuously augments its therapeutic efficacy, especially in tumor metastasis and recurrence. Exceptional multimodal imaging navigation has also been developed. Excellent theranostics performance was substantiated in diverse tumor models, implying that this synergistic strategy of phototheranostics and immunotherapy provides a paradigm shift in emerging CRISPR-mediated nanomedicines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


