发现从阿枯米星提取的强效 Kappa 阿片受体激动剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
Akuammicine (1) 是一种从 Picralima nitida 分离出来的生物碱,是卡巴阿片受体(κOR)的激动剂。为了建立这种结构独特的κOR 配体的结构-活性关系(SAR),我们合成了一系列半合成衍生物。通过评估这些衍生物激活κOR 和μ阿片受体(μOR)的能力,发现了关键的 SAR 趋势,并确定了具有更强κOR 效用的衍生物。最值得注意的是,芳基环 C10 位置的取代使κOR 的效力提高了 200 倍,对κOR 几乎具有完全的选择性。研究表明,精选出的最有效配体具有不同的将 β-Arrestin-2 募集到 κOR 的能力,这表明它们具有不同于彼此和现有 κOR 配体的信号转导特性。这些κOR激动剂的发现凸显了利用天然产物鉴定新型强效选择性配体的潜力,并为探究κOR提供了新的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
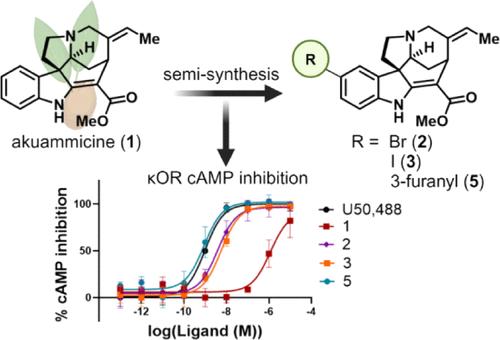
Discovery of Potent Kappa Opioid Receptor Agonists Derived from Akuammicine
Akuammicine (1), an alkaloid isolated from Picralima nitida, is an agonist of the kappa opioid receptor (κOR). To establish structure–activity relationships (SARs) for this structurally unique κOR ligand, a collection of semisynthetic derivatives was synthesized. Evaluating these derivatives for their ability to activate the κOR and mu opioid receptor (μOR) revealed key SAR trends and identified derivatives with enhanced κOR potency. Most notably, substitutions to the C10 position of the aryl ring led to a > 200-fold improvement in κOR potency and nearly complete selectivity for the κOR. A selection of the most potent ligands was shown to possess differing abilities recruitment of β-Arrestin-2 to the κOR, indicating they have distinct signaling properties from each other and existing κOR ligands. The discovery of these κOR agonists underscores the potential of using natural products to identify new classes of potent and selective ligands and provides new tools to probe the κOR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


