La0.4Sr0.6CoO3 催化乙苯无溶剂选择性氧化为苯乙酮:由 1O2 衍生的 -O2- 介导的一种新的活性氧转化机制
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
开发一种绿色、稳定、经济的异相催化剂,并阐明其催化机理,用于无溶剂条件下 C-H 键对羰基化合物的选择性氧化,具有重要的理论和实用价值。在此,我们采用溶胶-凝胶法合成了过氧化物催化剂,用于催化乙苯的选择性氧化。值得注意的是,La0.4Sr0.6CoO3-800(催化剂的煅烧温度为 800 °C)表现出了显著的功效,可将 73% 的乙苯转化为苯乙酮,选择性高达 93%。表征分析表明,锶的加入适度地破坏了包晶结构的内部平衡,导致氧空位增加,氧吸附能力增强。此外,电子顺磁共振和机理研究证明,催化剂表面的分子氧被转化为单线态氧(1O2)和超氧自由基阴离子(-O2-)。1O2 的存在极大地促进了 -O2- 的产生,从而有效地促进了乙苯的氧化。这项研究引入了一种新的活性氧(ROS)转化机制,为碳氢化合物的选择性氧化提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
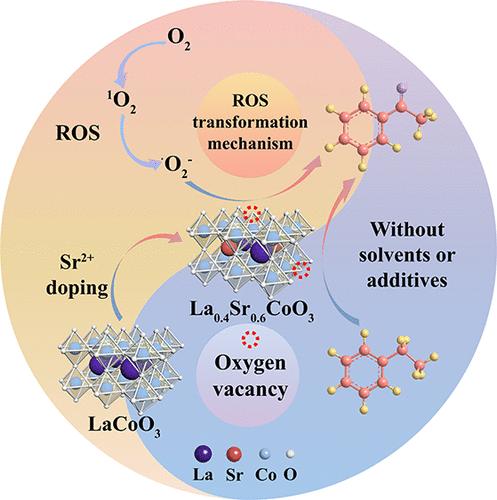
La0.4Sr0.6CoO3-Catalyzed Selective Oxidation of Ethylbenzene to Acetophenone without Solvent: A New Reactive Oxygen Species Transformation Mechanism Mediated by •O2– Derived from 1O2
Developing a green, stable, and cost-effective heterogeneous catalyst and clarifying its catalytic mechanism for the selective oxidation of C–H bonds without solvent to carbonyl compounds hold a significant theoretical and practical value. Herein, we synthesize perovskite catalysts using the sol–gel method to catalyze the selective oxidation of ethylbenzene. Notably, La0.4Sr0.6CoO3-800 (800 °C is the calcination temperature of the catalyst) demonstrates remarkable efficacy, converting 73% of ethylbenzene into acetophenone with a selectivity of 93%. Characterization analyses reveal that the incorporation of strontium moderately disrupts the internal balance of the perovskite structure, leading to increased oxygen vacancies and enhanced oxygen adsorption capacity. Moreover, electron paramagnetic resonance and mechanistic studies prove that molecular oxygen on the catalyst surface is converted to singlet oxygen (1O2) and superoxide radical anions (•O2–). The presence of 1O2 significantly aids in the production of •O2–, thereby effectively promoting the oxidation of ethylbenzene. This research introduces a new reactive oxygen species (ROS) transformation mechanism and provides valuable insights into the selective oxidation of hydrocarbons.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


