磺酰胺基苯基)喹啉-4-羧酸的合成和单胺氧化酶抑制特性
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过乙酰苯磺酰胺类化合物与几种取代的异汀类化合物的反应,我们合成了一系列 22 种一级磺酰胺类化合物,并对其中 16 种进行了 MAO-A 和 MAO-B 抑制活性测试。同时,为了使所得结构多样化,我们进行了脱羧以及羧酸的进一步官能化。研究结果表明,6-氯-2-(3-氨基磺酰基苯基)喹啉-4-甲酸甲酯能选择性地抑制 MAO-B,其 IC50 值为 13.7 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
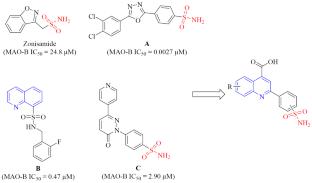
Synthesis and Monoamine Oxidase Inhibition Properties of (Sulfamoylphenyl)quinoline-4-carboxylic Acids
By reaction between acetylbenzenesulfonamides with several substituted isatins, we have synthesized a series of 22 primary sulfonamides, 16 of which were tested for inhibition activity against MAO-A and MAO-B. At the same time, in order to diversify the resulting structures, we have carried out decarboxylation as well as further functionalization at the carboxylic acid. The results of the study show that methyl 6-chloro-2-(3-sulfamoylphenyl)quinoline-4-carboxylate selectively inhibited MAO-B with a value of IC50 = 13.7 μM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


