从分子角度看 H2O-O2-Cl- 的吸附和反应对钢铁初始电化学腐蚀的影响
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
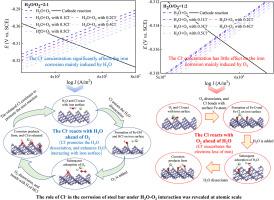
深入了解氯化物对铁初始腐蚀过程的影响,可以揭示 Cl 诱导钢加速腐蚀的详细机理。鉴于在实验中难以揭示 H2O-O2-Cl- 相互作用的复杂性,本研究采用 DFT 方法对 H2O、O2 和 Cl- 共吸附及相互作用对铁(100)表面电化学腐蚀过程的影响进行了分子研究,电子相关函数为 GGA-PW91。研究发现,H2O、O2 和 Cl- 的吸附都会促进铁的电化学腐蚀。铁表面的电化学腐蚀速率随着 Cl-/H2O 浓度比的增加而上升,随着 Cl-/O2 浓度比的增加而下降。过渡态搜索发现,Cl- 的存在有助于 H2O 在铁表面的解离,而 Cl- 与 O2 之间的相互作用则可以忽略不计。此外,还进一步研究了 Cl-、H2O 和 O2 与铁表面的连续反应。当 Cl- 先于 O2 与 H2O 反应时,Cl- 通过促进 H2O 的解离来促进铁的氧化。O2 和 Cl- 首先在铁表面发生共吸附,而 Cl- 则通过加剧铁表面失去电子来加速铁的氧化。这些发现有助于开发氯化物环境中钢铁的防腐蚀技术。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular insights into the effect of adsorption and reaction of H2O-O2-Cl- on initial electrochemical corrosion of steel
A deeper understanding of the effect of chloride on the process of iron initial corrosion could reveal the detailed mechanism of the Cl--induced acceleration corrosion of steel. In light of the difficulty to unravel the intricacies of H2O-O2-Cl- interactions in experiments, this study conducted the molecular study on the effect of H2O, O2 and Cl- coadsorption and interactions on the electrochemical corrosion process of Fe (100) surface by DFT method, and the electron-correlation functional is GGA-PW91. It is discovered that the adsorption of H2O, O2 and Cl- all promote the electrochemical corrosion of iron. The electrochemical corrosion rate of iron surface rises as the Cl-/H2O concentration ratio increasing, while decrease with the Cl-/O2 concentration ratio increasing. The transition state search found that the presence of Cl- assists the dissociation of H2O on the iron surface, while the interaction between Cl- and O2 could be ignored. Moreover, the successive reaction of Cl-, H2O and O2 with iron surface were further investigated. When the Cl- reacts with H2O ahead of O2, the Cl- promotes the iron oxidation via promoting the dissociation of H2O. While O2 and Cl- firstly coadsorb on iron surface, Cl- accelerates iron oxidation by exacerbating the iron surface losing electrons. These findings could facilitate the development of anti-corrosion techniques for steel in chloride environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


