三明治状层状金属硫化物离子交换剂对复杂环境中 Ni2+ 的高选择性捕获
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
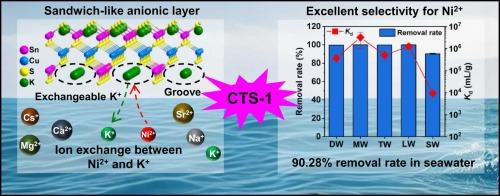
放射性镍离子(63Ni2+)的修复对公众健康和环境至关重要,但从海水等复杂环境中选择性地捕获 Ni2+ 却是一项挑战。金属硫化物离子交换剂(MSIEs)正在成为放射性核素的高效吸附剂;然而,对 MSIEs 选择性去除 Ni2+ 的研究仍处于起步阶段。本文首次采用水热法合成了具有独特夹层阴离子框架的层状金属硫化物 K2Cu2Sn2S6(CTS-1),为从复杂环境中选择性捕获 Ni2+ 提供了一种新方法。单晶结构分析证实了这种 "三明治 "式框架,其中一个[Cu-S]亚层被两个带有平行沟槽的[Sn-S]亚层夹住。电荷平衡的 K+ 离子位于这些沟槽中。由于其特殊的结构,CTS-1 对 Ni2+ 具有显著的吸附能力、快速的动力学特性(速率常数 k2 高达 7.26×10-2 g/(mg-min))、广泛的 pH 持久性(pH 值为 3-12 时的去除率为 97%)和高选择性(Ni2+ 与各种阳离子的分离系数为 700)。令人印象深刻的是,它能从多种复杂环境中高效去除 Ni2+,即使在海水(C0Ni ~5 mg/L)中也能达到 90.28% 的去除率。CTS-1 对环境友好,适合在固定床色谱柱中实际应用。此外,Ni2+ 离子是通过与 K+ 的离子交换捕获的,而高选择性则源于 S2- 对 Ni2+ 的强亲和力和结构内凹槽的捕获效应。总之,这项开创性的研究证明了三明治状层状 MSIE 对 Ni2+ 的高选择性捕获,可能会对开发高效的放射性核素清除剂有所启发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Highly selective capture of Ni2+ from complex environments by a sandwich-like layered metal sulfide ion exchanger
Radionickel ion (63Ni2+) remediation is critical for public health and the environment, but selectively capturing of Ni2+ from complex environments like seawater presents a challenge. Metal sulfide ion exchangers (MSIEs) are emerging as efficient adsorbents for radionuclides; however, the study of MSIEs for selectively removing Ni2+ is still in its infancy. Herein, the layered metal sulfide K2Cu2Sn2S6 (CTS-1) with a unique sandwich-like anionic framework was synthesized by the hydrothermal method for the first time, representing a novel approach in the selective capture of Ni2+ from complex environments. Single-crystal structural analysis confirmed the sandwich-like framework, in which a [Cu-S] sublayer is sandwiched by two [Sn-S] sublayers with parallel grooves. The charge-balancing K+ ions are located within these grooves. Due to its special structure, CTS-1 exhibits remarkable adsorption capacities for Ni2+ with rapid kinetics (a high rate constant k2 of 7.26×10−2 g/(mg·min)), broad pH durability (removal rates >97% at pH 3–12), and high selectivity (separation factors for Ni2+ >700 against various cations). Impressively, it can efficiently remove Ni2+ from multiple complex environments, achieving a 90.28% removal rate even in seawater (C0Ni ~5 mg/L). CTS-1 is environmentally friendly and suitable for use in fixed-bed columns for the practical application. Moreover, Ni2+ ions are captured through ion exchange with K+, and the high selectivity stems from the strong affinity of S2− for Ni2+ and the trapping effect of the grooves within the structure. In summary, this pioneering study demonstrates the highly selective capture of Ni2+ by a sandwich-like layered MSIE, potentially inspiring the development of efficient scavengers for radionuclides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


