含无机盐和有机盐的聚合物电解质用于钠离子超级电容器
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
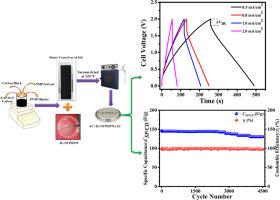
含有共聚物 PVdF-HFP、10 wt.%无机盐{双(三氟甲烷磺酰基)亚胺钠(NaTFSI)}和 70 wt.%有机盐{1-丁基-1-甲基吡咯烷鎓双(三氟甲烷磺酰基)亚胺([BMPYr] [TFSI])}的优化高离子导电聚合物电解质膜已被用于制造钠离子超级电容器。优化后的电解质薄膜具有最高的室温(RT)离子电导率(∼0.87 mS/cm)、优异的机械稳定性和较大的工作电位窗口(∼5.5 V)。利用这种薄膜和活性碳电极(ACE (AC∼0.8 mg/cm2)),电化学双层电容器(EDLC)/纳离子超级电容器已经制作完成。该电池的体电阻为 22.9 Ω,表明电极-电解质接触良好。EDLC 电池的循环伏安 (CV) 结果显示几乎呈矩形,这证明了其电容行为。在不同的等电流密度(分别为 0.5、0.8、1.0 和 2.0 mA/cm2)下,所制造的钠离子超级电容器的比容量分别为 173 F/g、151.91 F/g、145.32 F/g 和 122.33 F/g,库仑效率从 97.6% 到 99.9% 不等,在 1 mA/cm2 下可循环使用 4500 次。通过循环伏安法获得的 EDLC 电池的比电容值与通过电静态充放电(GCD)测量获得的值十分吻合。此外,在 1 mA/cm2 的条件下,循环性能基本稳定,最高可达 2500 次,之后比电容值(CSP/CD)略有下降,最高可达 4500 次。CSP/CD 值的降低可能是由于固体电解质界面 (SEI) 层的厚度增加及其相应的界面电阻所致。在 0.5 mA/ cm2 等电流密度下,第一个循环的最大比能量和功率密度分别为 31.52 Wh/Kg 和 ∼472.8 kW/Kg。使用 ACE(AC∼1.6 mg/cm2)时,在 0.5 mA/cm2 的条件下,所制造的钠离子 EDLC 电池的比电容值达到最大值 ∼72.6 F/g,库仑效率在 96.5 % 至 99.5 % 之间。在第一个循环中,能量和功率密度均有所提高(分别为 40.31 Wh/Kg 和 499.12 kW/Kg),并显示出稳定的循环能力,最高可达 3000 个循环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Polymer electrolyte with inorganic and organic salts for Na-Ion supercapacitor
The optimized high ion conducting polymer electrolyte film containing copolymer PVdF-HFP, 10 wt.% inorganic salt {sodium bis(trifluoromethane sulfonyl)imide (NaTFSI)} and 70 wt.% organic salt {1-butyl-1-methypyrrolidinium bis(trifluoromethane sulfonyl) imide ([BMPYr] [TFSI])} have been used for the fabrication of Na-ion supercapacitor. The optimized composition of the electrolyte film possesses maximum room temperature (RT) ionic conductivity (∼0.87 mS/cm), excellent mechanical stability and large operating potential window (∼5.5 V). Using this film and activated carbon electrode (ACE (AC∼0.8 mg/cm2)), electrochemical double layer capacitor (EDLC)/Na-ion supercapacitor has been fabricated. The bulk resistance of this cell is found to be 22.9 Ω which is an evidence of good electrode-electrolyte contact. The cyclic voltammetric (CV) results of the EDLC-cell displays almost rectangular shape which demonstrates their capacitive behavior. The fabricated Na-ion supercapacitor has delivered specific capacities of ∼173 F/g, 151.91 F/g, 145.32 F/g and 122.33 F/g at different areal current densities (∼0.5, 0.8, 1.0 and 2.0 mA/cm2, respectively) along with the coulombic efficiency ranging from 97.6% to 99.9% upto 4500 cycles at 1 mA/cm2. The obtained value of the specific capacitance(s) of the EDLC cell from cyclic voltammetry is in good agreement with the value obtained from galvanostatic charge-discharge (GCD) measurements. Also, a nearly stable cycling performance has been obtained at 1 mA/cm2 upto 2500 cycles and after that the value of the specific capacitance (CSP/CD) decreases slightly upto 4500 cycles. This decrease in CSP/CD value may be because of increased thickness of solid electrolyte interface (SEI) layer and its corresponding interfacial resistance. The maximum specific energy and power density at 0.5 mA/ cm2 areal current density for first cycle are 31.52 Wh/Kg and ∼472.8 kW/Kg, respectively. On using ACE having AC∼1.6 mg/cm2, the fabricated Na-ion EDLC-cell has given maximum value of specific capacitance as ∼72.6 F/g at 0.5 mA/cm2 alongwith coulombic efficiency in the range of 96.5 % to 99.5 %. For the first cycle, the energy as well power density increase (∼40.31 Wh/Kg and ∼499.12 kW/Kg, respectively) and show stable cyclability upto 3000 cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


