胺功能化无定形 SiO2-Al2O3 吸附剂的二氧化碳捕集性能:对吸附剂酸性的见解
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
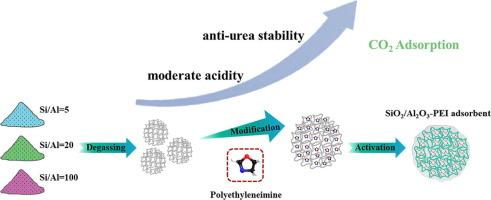
胺功能化吸附剂具有高选择性和广泛的应用范围,因此在二氧化碳捕集方面具有相当大的潜力。然而,它们容易受到二氧化碳引起的化学失活的影响。尽管研究人员努力合成具有丰富酸性位点的吸附剂来吸附胺并提高其稳定性,但关于表面酸性的变化如何影响所得吸附剂的二氧化碳吸附性能的信息仍然很少。为此,我们合成了表面酸度不同的多孔无定形 SiO2-Al2O3 并用聚乙烯亚胺(PEI)浸渍。然后,我们研究了在不同温度、再生气氛和湿度条件下的二氧化碳吸附性能。结果表明,最佳吸附温度为 75 ℃,预处理温度为 140 ℃。在这些条件下,Si/Al = 20-60 样品的捕获能力最高,约为 142.6 mg/g。事实证明,阿夫拉米模型最适合用于拟合各种吸附剂的二氧化碳吸附数据,从而准确评估整个动态吸附过程。然而,循环稳定性测试表明,在 SiO2-Al2O3 吸附剂中,Si/Al=5-50 在干燥和潮湿条件下的稳定性都是最高的,这是因为它对尿素形成的抵抗力更强。利用傅立叶变换红外光谱、固态核磁共振和 XPS 分析,我们发现 SiO2-Al2O3 表面中等强度的路易斯酸位点密度在抗尿素形成方面起着关键作用,因为它能诱导 PEI 与多孔支撑物之间发生最高程度的交联反应。这一突破为了解支撑材料的表面酸性如何影响用于捕获二氧化碳的固体胺吸附剂的稳定性提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CO2 capture performance of amine-functionalized amorphous SiO2-Al2O3 adsorbent: Insights into the support acidity
Amine-functionalized adsorbents possess considerable potential for CO2 capture due to their high selectivity and versatility across a range of applications. However, they are susceptible to CO2-induced chemical deactivation. Despite research efforts to synthesize supports with abundant acid sites to accommodate amines and enhance their stability, information remains sparse on how changes in surface acids impact the CO2 adsorption performance of the resulting adsorbents. In this context, we synthesized porous amorphous SiO2-Al2O3 with varying surface acidity, and impregnated with polyethylenimine (PEI). We then investigated the CO2 adsorption performance under different temperatures, regeneration atmospheres, and humidity levels. The results indicated an optimal adsorption temperature of 75 °C and a pre-treatment temperature of 140 °C. Under these conditions, the Si/Al = 20–60 sample demonstrated the highest capture capacity, approximately 142.6 mg/g. The Avrami model proved most suitable for fitting CO2 adsorption data across various adsorbents, providing an accurate assessment of the entire dynamic adsorption process. However, cycle stability tests revealed that Si/Al = 5–50 had the highest stability among the SiO2-Al2O3 adsorbents in both dry and humid conditions, due to its superior resistance to urea formation. Utilizing FT-IR, solid-state NMR, and XPS analysis, we discovered that the density of moderately strong Lewis acid sites on the surface of SiO2-Al2O3 plays a crucial role in resisting urea formation, as it induces the highest degree of cross-linking reaction between PEI and the porous supports. This breakthrough offers new insights into how the surface acidity of support materials influences the stability of solid amine adsorbents for CO2 capture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


