通过接枝功能性有机分子利用金属有机框架的节点进行协同铀提取
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
高效消除铀是核能发展方面的一个研究热点。金属螯合剂和多孔材料是从废水中回收和分离铀的两项前沿技术。然而,将金属螯合剂的金属配位功能转移到化学性质稳定的宿主材料上的成功案例十分有限。本文采用简单的溶剂辅助配体加入法制备了草氨酸(OxA)和甘氨酸(Gly)功能化的 MOF-808,并将其用于铀的去除。MOFs的有序多孔结构提供了快速扩散通道,而在Zr6节点上引入氨基酸则赋予MOF-808通道更多的功能基团,使其具有强结合能力和高亲水性。在 pH = 5 条件下,MOF-808@OxA 比 MOF-808@Gly 和原始 MOF-808 具有更高的消除能力(qmax = 370.76 mg-g-1)、更快的消除速度(∼40 分钟)和更高的选择性。特别是密度泛函理论计算表明,由于 C = O 和 -NH2 基团的协同作用,MOF-808@OxA 与 MOF-808@Gly 相比对铀具有更强的亲和力。因此,这项研究为改造 MOFs 提供了一种可行的策略,并为基于 MOF 的材料消除废水中的铀提供了广阔的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
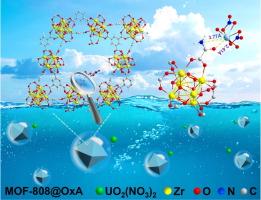
Exploiting the nodes of metal-organic framework by grafting functional organic molecules for synergistic uranium extraction
High-efficiency elimination of uranium was a research hotspot from the aspect of nuclear energy development. Metal chelators and porous materials were two cutting-edge technologies for the recovery and separation of uranium from wastewater. However, there was only limited success in transferring the metal coordination function of metal chelators to chemically stable host materials. Herein, oxamic acid (OxA) and glycine (Gly) functionalized MOF-808 were prepared by a simple solvent-assisted ligand incorporation method and used for uranium removal. The ordered porous structure of MOFs provided rapid diffusion channels, and the introduction of amino acids on Zr6 nodes endowed MOF-808 channels more functional groups with strong binding ability and high hydrophily. The MOF-808@OxA exhibited higher elimination ability (qmax = 370.76 mg·g−1), rapider elimination rate (∼40 min), and higher selectivity than those of MOF-808@Gly and original MOF-808 at pH = 5. Particularly, density functional theory calculations revealed that MOF-808@OxA had a stronger affinity for uranium compared to MOF-808@Gly due to the synergistic effect of C = O and –NH2 groups. Thus, this study provided a feasible strategy for modifying MOFs and a promising prospect for MOF-based materials to eliminate uranium from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


