均匀乳杆菌通过靶向内质网应激介导的铁蛋白沉积,改善饮食引起的炎症性加剧结肠炎
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
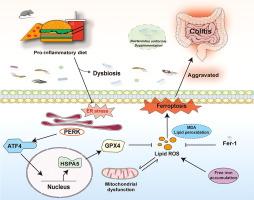
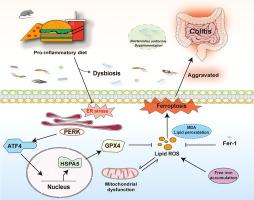
导言:促炎性饮食与炎症性肠病(IBD)的风险和进展呈正相关。本研究旨在阐明促炎饮食介导结肠炎的机制,并探索潜在的干预策略。随后,使用 2.5 % 右旋糖酐硫酸钠化学诱导结肠炎。通过评估体重、病理评分、免疫反应和粘膜屏障功能来评估肠道炎症。应用肠道组织转录组学、粪便微生物组分析和血清代谢组学来确定饮食-微生物-宿主之间的相互作用。此外,还对 32 名克罗恩病患者的膳食炎症指数(DII)评分和肠道标本进行了评估。结果前炎性饮食会诱发小鼠低度肠道炎症,并通过激活内质网应激介导途径中与谷胱甘肽过氧化物酶 4 相关的铁氧化酶来加重结肠炎。铁前列素-1 治疗可逆转这些影响。此外,促炎性饮食会通过调节肠道微生物群和代谢物引发结肠炎。值得注意的是,补充均匀酵母菌能抑制内质网应激介导的铁蛋白沉积,从而改善促炎性饮食导致的结肠炎。结论前炎性饮食通过靶向内质网应激介导的铁蛋白沉积,可能以肠道微生物群依赖的方式驱动结肠炎。限制前炎性饮食和基于微生物的疗法可能是预防和治疗 IBD 的有效策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bacteroides uniformis ameliorates pro-inflammatory diet-exacerbated colitis by targeting endoplasmic reticulum stress-mediated ferroptosis
Introduction
A pro-inflammatory diet is positively associated with the risk and progression of inflammatory bowel diseases (IBD). Recently, ferroptosis has been observed in patients with different dietary patterns-associated intestinal inflammation, while the mechanisms underlying the effects of a pro-inflammatory diet and whether it mediates ferroptosis are unknown.
Objectives
This study aims to elucidate the mechanisms underlying pro-inflammatory diet-mediated colitis and explore potential intervention strategies.
Methods
Mice were fed a dietary inflammatory index-based pro-inflammatory diet for 12 weeks. Subsequently, colitis was chemically induced using 2.5 % dextran sulfate sodium. The body weight, pathological score, immune response and mucosal barrier function were evaluated to assess intestinal inflammation. Intestine tissue transcriptomics, fecal microbiome analysis and serum metabolomics were applied to identify diet–microbe–host interactions. Additionally, the dietary inflammatory index (DII) scores and intestinal specimens of 32 patients with Crohn’s disease were evaluated. The biological functions of Bacteroides uniformis were observed in vitro and in vivo.
Results
Pro-inflammatory diet induces low-grade intestinal inflammation in mice and exacerbates colitis by activating glutathione peroxidase 4-associated ferroptosis in the endoplasmic reticulum stress-mediated pathway. These effects are reversed by ferrostatin-1 treatment. Additionally, the pro-inflammatory diet triggers colitis by modulating the gut microbiota and metabolites. Notably, supplementation with B. uniformis improves the pro-inflammatory diet-aggravated colitis by inhibiting endoplasmic reticulum stress-mediated ferroptosis. Moreover, B. uniformis is non-enterotoxigenic and non-enteroinvasive in co-cultures with intestinal epithelial cells.
Conclusions
Pro-inflammatory diet drives colitis by targeting endoplasmic reticulum stress-mediated ferroptosis, possibly in a gut microbiota-dependent manner. Pro-inflammatory diet restriction and microbial-based therapies may be effective strategies for preventing and treating IBD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


