机械化学法制备的掺镍草酸零价铁催化生成吸附原子 H 以降解 2,2',4,4'-四溴二苯醚
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
在多溴联苯醚(PBDEs)的同族系列中,毒性较高的低溴联苯醚的脱溴仍然是一项挑战。纳米零价铁(nZVI)在多溴联苯醚脱溴方面已得到广泛研究,但其固有的直接电子转移机制对低溴联苯醚的脱溴效率较低,而且存在制备成本高的问题。在这项工作中,我们采用低成本球磨法合成了掺杂镍的草酸盐亚微米 ZVI(FeOXbm/Ni)。在四氢呋喃(THF)/水中,FeOXbm/Ni 对 2,2',4,4'-四溴二苯醚(BDE-47)的脱溴速率常数为 0.48 天-1。在水中,FeOXbm/Ni 对 BDE-47 的脱溴速度更快(0.98 天-1),完全脱溴产物二苯醚的产量达到 76.71%。在实际地下水中,FeOXbm/Ni 对 BDE-47 也表现出很高的反应活性,其速率常数为 0.33 天-1。动力学实验、淬火实验和降解途径表明,原子氢对 C-Br 键的攻击是主要的降解机制。电化学分析进一步表明,Ni0 位点可将氢裂解为吸收的原子氢(H*ABS)和吸附的原子氢(H*ADS),其中 H*ADS 起主要作用。这些发现为推进 ZVI 的大规模应用提供了宝贵的见解,并为彻底修复多溴联苯醚污染提供了有前景的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
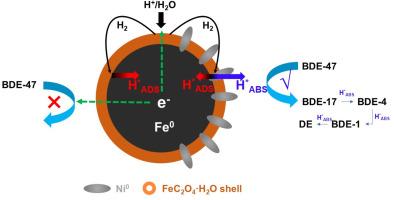
Catalytic generation of adsorbed atomic H for degradation of 2,2',4,4'-tetrabromodiphenyl ether by mechanochemically prepared Ni-doped oxalated zero-valent iron
In the homologous series of polybrominated diphenyl ethers (PBDEs), the debromination of low-brominated diphenyl ethers with higher toxicity remains a challenge. Nano zero-valent iron (nZVI) has been extensively studied for the debromination of PBDEs, but its inherent direct electron transfer mechanism is less efficient for low-brominated diphenyl ethers, and there are issues with high preparation costs. In this work, we synthesize Ni-doped oxalated submicron ZVI (FeOXbm/Ni) using a low-cost ball-milling method. FeOXbm/Ni exhibits a debromination rate constant of 0.48 day-1 for 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) in tetrahydrofuran (THF)/water. The debromination rate of FeOXbm/Ni for BDE-47 in water is even faster (0.98 day-1), with the yield of the complete debromination product, diphenyl ether, reaching 76.71%. In real groundwater, FeOXbm/Ni also shows high reactivity toward BDE-47, with a rate constant of 0.33 day-1. Kinetic experiments, quenching experiments, and degradation pathway indicate that the attack of atomic hydrogen on C-Br bonds is the primary degradation mechanism. Electrochemical analysis further show that Ni0 sites could cleave hydrogen into absorbed atomic hydrogen (H*ABS) and adsorbed atomic hydrogen (H*ADS), with H*ADS playing the main role. These findings contribute valuable insights into advancing the large-scale application of ZVI and offer promising strategies for thorough remediation of PBDEs pollution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


