AMPLATZER Amulet 左房阑尾封堵器随机对照试验的 5 年结果
IF 22.3
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
背景Amulet IDE 试验(AMPLATZER Amulet 左心房阑尾封堵器[LAAO] 研究性设备豁免[IDE] 试验)评估了 Amulet 封堵器(雅培)在非瓣膜性心房颤动患者中的安全性和有效性。Amulet IDE 试验是最大的 LAAO 随机试验,比较了 Amulet 封堵器和 Watchman 2.5 设备(波士顿科学公司)。方法Amulet IDE 试验的入组患者为中风或全身性栓塞的高危人群,定义为 CHADS2 评分≥2 或 CHA2DS2-VASc 评分≥3。结果 共有1878名患者接受了随机治疗,其中1833名接受了装置植入尝试(n = 917,Amulet闭塞器;n = 916,Watchman装置)。在每次随访中,Amulet 封堵器组患者无 OAC 的比例明显更高,在最后一次 5 年随访中,Amulet 和 Watchman 装置组无 OAC 的比例分别为 94.0% 和 90.9%(P = 0.009)。Amulet 装置组和 Watchman 装置组的 5 年临床结果相似,包括缺血性卒中或全身性栓塞的复合死亡率(7.4% vs 7.1%;P = 0.851)、卒中、全身性栓塞或心血管死亡的复合死亡率(20.3% vs 20.7%; P = 0.666)、大出血(20.1% vs 20.0%; P = 0.882)、心血管(CV)死亡(14.3% vs 15.4%; P = 0.429)和全因死亡(28.7% vs 31.1%; P = 0.217)。5 年的年化缺血性脑卒中发生率较低,Amulet(1.6%/年)和 Watchman(1.6%/年)装置的发生率相同。与使用 Watchman 装置的患者相比,使用 Amulet 闭塞器的患者中风的严重程度较低(n = 38,非致残;n = 11,致残;n = 11,致命;n = 12,未知)(n = 19,非致残;n = 22,致残;n = 17,致命;n = 10,未知)。此外,与使用 Amulet 封堵器的患者(n = 31)相比,使用 Watchman 设备的患者(n = 63)在发生脑卒中事件和心血管疾病死亡之前更常出现设备因素(设备相关血栓或设备周围泄漏≥3 mm)。结论最大的 LAAO 随机临床试验的 5 年结果表明,Amulet 封堵器和 Watchman 2.5 设备具有长期安全性和有效性。双密封 Amulet 封堵器可减少与心房颤动相关的血栓栓塞事件,同时无需长期使用 OAC。(AMPLATZER Amulet 左心房阑尾封堵器[LAAO]研究性设备豁免[IDE]试验[Amulet IDE 试验];NCT02879448)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
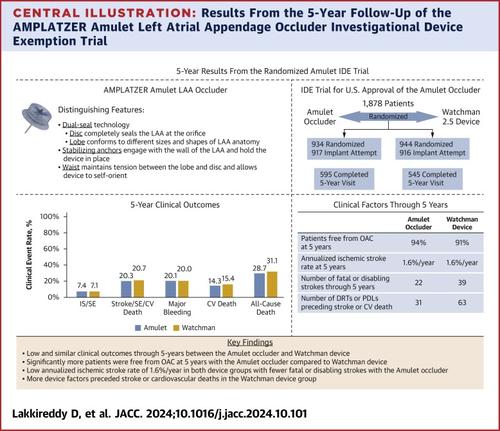
5-Year Results From the AMPLATZER Amulet Left Atrial Appendage Occluder Randomized Controlled Trial
Background
The Amulet IDE trial (AMPLATZER Amulet Left Atrial Appendage Occluder [LAAO] Investigational Device Exemption [IDE] Trial) evaluated the safety and effectiveness of the Amulet occluder (Abbott) in patients with nonvalvular atrial fibrillation. The Amulet IDE trial is the largest randomized LAAO trial, comparing the Amulet occluder with the Watchman 2.5 device (Boston Scientific).
Objectives
This analysis presents the 5-year results from the trial comparing the 2 devices head to head.
Methods
Patients enrolled in the Amulet IDE trial were at a high risk of stroke or systemic embolism defined as a CHADS2 score ≥2 or CHA2DS2-VASc score ≥3. Oral anticoagulation (OAC) use and key clinical outcomes are presented through 5 years.
Results
A total of 1,878 patients were randomized, with 1,833 undergoing a device implantation attempt (n = 917, Amulet occluder; and n = 916, Watchman device). A significantly higher percentage of patients were free of OAC in the Amulet occluder group at each follow-up visit, with 94.0% and 90.9% free of OAC at the last 5-year follow-up visit in the Amulet and Watchman device groups, respectively (P = 0.009). The 5-year clinical outcomes were similar between the Amulet and Watchman devices, including the composite of ischemic stroke or systemic embolism (7.4% vs 7.1%; P = 0.851), the composite of stroke, systemic embolism, or cardiovascular death (20.3% vs 20.7%; P = 0.666), major bleeding (20.1% vs 20.0%; P = 0.882), cardiovascular (CV) death (14.3% vs 15.4%; P = 0.429), and all-cause death (28.7% vs 31.1%; P = 0.217). Annualized ischemic stroke rates at 5 years were low and the same for Amulet (1.6%/y) and Watchman (1.6%/y) devices. Strokes in patients with the Amulet occluder were less severe (n = 38, nondisabling; n = 11, disabling; n = 11, fatal; n = 12, unknown) than strokes in patients with the Watchman device (n = 19, nondisabling; n = 22, disabling; n = 17, fatal; n = 10, unknown). Moreover, device factors (device-related thrombus or peridevice leak ≥3 mm) preceded stroke events and CV deaths more frequently in patients with the Watchman device (n = 63) compared with patients with the Amulet occluder (n = 31).
Conclusions
The 5-year outcomes from the largest randomized LAAO clinical trial demonstrated the long-term safety and effectiveness of the Amulet occluder and Watchman 2.5 devices. The dual-seal Amulet occluder reduces atrial fibrillation–related thromboembolic events while eliminating the need for long-term OAC. (AMPLATZER Amulet Left Atrial Appendage Occluder [LAAO] Investigational Device Exemption [IDE] Trial [Amulet IDE trial]; NCT02879448)
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
42.70
自引率
3.30%
发文量
5097
审稿时长
2-4 weeks
期刊介绍:
The Journal of the American College of Cardiology (JACC) publishes peer-reviewed articles highlighting all aspects of cardiovascular disease, including original clinical studies, experimental investigations with clear clinical relevance, state-of-the-art papers and viewpoints.
Content Profile:
-Original Investigations
-JACC State-of-the-Art Reviews
-JACC Review Topics of the Week
-Guidelines & Clinical Documents
-JACC Guideline Comparisons
-JACC Scientific Expert Panels
-Cardiovascular Medicine & Society
-Editorial Comments (accompanying every Original Investigation)
-Research Letters
-Fellows-in-Training/Early Career Professional Pages
-Editor’s Pages from the Editor-in-Chief or other invited thought leaders

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


