弓形虫 GRA15 在树突状细胞上的表达会抑制 B 细胞分化和抗体生成。
IF 1.5
4区 医学
Q3 PARASITOLOGY
引用次数: 0
摘要
据了解,弓形虫(T. gondii)中一种名为 GRA15 的致密颗粒蛋白可通过激活 NF-κB 支持宿主的先天性免疫反应。然而,人们对 GRA15 对寄生虫的益处知之甚少。通过研究 GRA15 在宿主与寄生虫相互作用中的作用,我们发现淋球菌中的 GRA15 抑制了宿主的获得性免疫反应。与Δgra15淋球菌相比,野生型寄生虫感染C57BL/6小鼠后,淋球菌抗体滴度较低,浆细胞计数也较低。为了确定 GRA15 在哪些宿主细胞中起抑制抗体产生的作用,我们产生了在特定细胞系中表达 GRA15 的条件性基因敲入小鼠。在感染Δgra15淋球菌后,条件性基因敲入小鼠的巨噬细胞中抗淋球菌抗体并没有减少,而条件性基因敲入小鼠(CD11c-Cre/GRA15cKI)的树突状细胞中淋球菌抗体的产生受到抑制。与对照组相比,用卵清蛋白(OVA)免疫的CD11c-Cre/GRA15cKI小鼠的抗OVA抗体滴度降低。此外,CD11c-Cre/GRA15cKI 中的 OVA 抗原特异性 T 细胞数量也有所减少。这些数据表明,树突状细胞中的GRA15抑制了T细胞介导的体液免疫。这些发现可能与 GRA15 的病理意义有关,并有助于弓形虫疫苗的生产。本文章由计算机程序翻译,如有差异,请以英文原文为准。
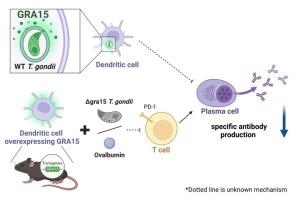
Toxoplasma GRA15 expression on dendritic cells inhibits B cell differentiation and antibody production
One of the dense granule proteins named GRA15 in Toxoplasma gondii (T. gondii), is known to support an innate immune response in host through activation of NF-κB. However, little is known about advantages of GRA15 for parasites. By examining the role of GRA15 in the host-parasite interactions, it was clarified that GRA15 in T. gondii suppressed acquired immune responses in host. Wild-type parasite infection to C57BL/6 mice resulted in lower titers of T. gondii antibody and lower plasma cell counts compared to Δgra15 T. gondii. To identify host cells in which GRA15 acts to suppress antibody production, we generated conditional knock-in mice that express GRA15 in specific cell lineages. Anti-T. gondii antibodies were not reduced in macrophages of conditional knock-in mice after infection with Δgra15 T. gondii, while the production of T. gondii antibody was suppressed in dendritic cells of the conditional knock-in mice (CD11c-Cre/GRA15cKI). In the CD11c-Cre/GRA15cKI immunized with ovalbumin (OVA), the titers of anti-OVA antibody were reduced compared to control mice. Furthermore, the number of OVA antigen-specific T cells was also decreased in CD11c-Cre/GRA15cKI. These data showed that GRA15 in dendritic cells suppressed T cell-mediated humoral immunity. These findings might implicate the pathological significance of GRA15 and facilitate Toxoplasma vaccines production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Parasitology International
医学-寄生虫学
CiteScore
4.00
自引率
10.50%
发文量
140
审稿时长
61 days
期刊介绍:
Parasitology International provides a medium for rapid, carefully reviewed publications in the field of human and animal parasitology. Original papers, rapid communications, and original case reports from all geographical areas and covering all parasitological disciplines, including structure, immunology, cell biology, biochemistry, molecular biology, and systematics, may be submitted. Reviews on recent developments are invited regularly, but suggestions in this respect are welcome. Letters to the Editor commenting on any aspect of the Journal are also welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


