利用代谢组学、基因组挖掘和生物评估的综合工作流程揭示了内生青霉 setosum 产生的含氧硫抗隐球菌二酮哌嗪。
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
隐球菌病是一种真菌感染,其治疗通常依赖于旧的抗真菌药物,但由于微生物的耐药性非常普遍,因此通常会导致高毒性和低效率等缺点。发现新的抗真菌剂迫在眉睫,而对尚未充分开发的天然产物(NP)的研究可以产生必要的成果,指导发现新的原型,以开发抗隐球菌分子。在这种情况下,一种涉及 P. setosum CMLD 18 的代谢组数据分析、生物评估和基因组挖掘的综合策略揭示了内生菌生物合成含双(甲硫基)氧杂环庚烷的二酮哌嗪衍生物--双硫代双(甲硫基)乙酰aranotine (1)。该分子对新变形杆菌的最低抑制浓度(MIC)值为 0.125 mM。研究还发现了 P. setosum 中负责生物合成(1)的相应生物合成基因簇(BGC)。此外,还检测到了(1)的其他假定类似物,这表明该微生物可能是抗隐球菌分子的重要来源,有待进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
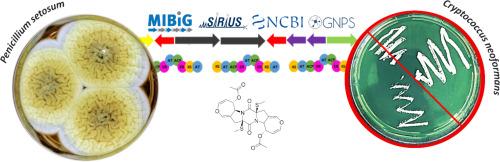
Integrated workflows using metabolomics, genome mining, and biological evaluation reveal oxepine‑sulfur-containing anti-cryptococcal diketopiperazine produced by the endophyte Penicillium setosum
Cryptococcosis is a fungal infection for which treatment relies on old antifungal agents usually leading to drawbacks such as high toxicity and mainly low efficiency since drug resistance of microorganisms is strongly widespread. The discovery of new antifungal agents is urgent and investigations about underexplored Natural Product (NP) can yield the necessary outcomes to guide the discovery of new prototypes to anti-cryptococcal molecules development. In this scenario, an integrated strategy involving metabolomic data analysis, biological assessement and genome mining of P. setosum CMLD 18, revealed the biosynthesis of bis(methyl-sulfanyl) oxepine-containing diketopiperazine derivative, the bisdethiobis(methylthio)acetylaranotine (1) by the endophyte. The molecule showed a minimum inhibitory concentration (MIC) value of 0.125 mM when tested against C. neoformans. Evidence about the corresponding biosynthetic gene cluster (BGC) responsible for the biosynthesis of (1) in P. setosum were found. Moreover, other putative analogues of (1) were also detected, suggesting this microorganism may represent an important source of likely anti-cryptococcal molecules to be further investigated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


