磁性卡拉胶接枝聚丙烯酰胺纳米复合材料用于吸收水生系统中的阳离子染料。
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-11-16
DOI:10.1016/j.ijbiomac.2024.137796
引用次数: 0
摘要
本研究采用原位生长法,制造出了 k 卡拉胶/聚丙烯酰胺@磁铁矿纳米复合材料(NCs)(k-Car/PAM@磁铁矿 NCs)。以过硫酸铵(APS)为引发剂,通过自由基聚合对丙烯酰胺(PAm)进行接枝聚合,从而对 k卡拉胶(k-Car)骨架进行改性。N'-N'亚甲基双丙烯酰胺(MBAA)被用作交联剂,磁性纳米粒子(MNPs)被加入其中以赋予其磁性。通过各种分析,包括傅立叶变换红外光谱(FT-IR)、有限电子显微镜(FESEM)、电致发光(EDS)、XRD、VSM、BET、TGA 和 Zeta,证实了 k-Car/PAM@Magnetite NCs 的成功合成。通过优化 pH 值、吸附剂用量、搅拌时间和初始染料浓度等实验参数,评估了 NCs 去除废水中亚甲基蓝(MB)和罗丹明 B(RhB)的吸附效果。根据朗穆尔等温线(Qmax),在使用 0.004 克 NCs 的情况下,NCs 对 MB 和 RhB 的最大吸附量分别为 204.082 毫克/克和 200 毫克/克。从热力学参数可以看出,吸附是一个自发且有利的内热过程。k-Car/PAM@Magnetite NCs 还具有出色的再生能力,在连续三次吸附-解吸循环后仍能保持较高的吸附效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
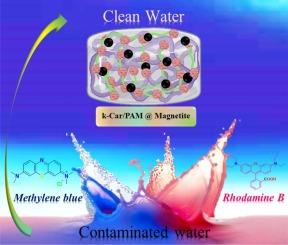
Magnetic carrageenan gum-grafted-polyacrylamide nanocomposite for uptake of cationic dyes from the aquatic systems
Using an ex-situ growth approach, this research fabricated k Carrageenan/Polyacrylamide@magnetite nanocomposites (NCs), (k-Car/PAM@Magnetite NCs). The k-Carrageenan (k-Car) backbone was modified through graft polymerization of acrylamide (PAm) via radical polymerization using ammonium persulfate (APS) as the initiator. N'-N' methylenebisacrylamide (MBAA) was used as the cross-linking agent, and magnetic nanoparticles (MNPs) were incorporated to impart magnetic properties. The successful synthesis of the k-Car/PAM@Magnetite NCs was confirmed through various analyses, including FT-IR, FESEM, EDS, XRD, VSM, BET, TGA, and Zeta. The adsorption effectiveness of the NCs for removing methylene blue (MB) and rhodamine B (RhB) from wastewater was evaluated by optimizing experimental parameters such as pH, adsorbent dosage, agitation time, and initial dye concentration. The NCs demonstrated a maximum adsorption capacity according to the Langmuir Isotherm (Qmax) of 204.082 mg. g−1 for MB and 200 mg.g−1 for RhB using 0.004 g of NCs.The adsorption data fit the Freundlich non-linear isotherm model well, and the kinetic data followed a pseudo-second-order (PSO) model. It is evident from the thermodynamic parameters that adsorption is an endothermic process that is spontaneous and favourable. The k-Car/PAM@Magnetite NCs also exhibited excellent regeneration capabilities, maintaining high adsorption efficiency after three consecutive adsorption-desorption cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


