通过铁催化的腈与亚磺酰胺的转移反应合成亚磺酰胺。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究报道了一种铁催化的腈转移反应,用于从现成的亚磺酰胺中快速合成亚磺酰胺类化合物。该方法的特点是条件温和、反应时间短、底物范围广,可以制备出多种亚氨基磺胺,而且收率良好甚至极佳。通过将其转化为其他有价值的硫(VI)化合物,如磺化二亚胺酰氟化物、磺化亚胺酯和磺化亚酰胺,进一步证明了亚磺脒产品的合成用途。开发不对称变体的初步工作显示了适度的对映选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
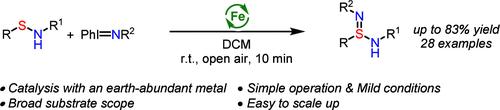
Synthesis of Sulfinamidines via Iron-Catalyzed Nitrene Transfer Reaction with Sulfenamides.
An iron-catalyzed nitrene transfer reaction for the rapid synthesis of sulfinamidines from readily available sulfenamides is reported. This method features mild conditions, short reaction times, and a broad substrate scope, allowing the preparation of a variety of sulfinamidines in good to excellent yields. The synthetic utility of the sulfinamidine products was further demonstrated through their conversion to other valuable sulfur(VI) compounds, such as sulfondiimidoyl fluorides, sulfinamidiate esters, and sulfonimidamides. Preliminary efforts in the development of an asymmetric variant showed moderate enantioselectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


