由固体结合肽介导的石墨上 Nadh 依赖性二氧化碳还原酶电容电催化界面。
IF 9.7
1区 环境科学与生态学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
将非本地石墨特异性肽(Gr;IMVTESSDYSSY)作为分子粘合剂引入改性自白色念珠菌(Candida boidinii)的 NAD+/NADH 依赖性二氧化碳还原酶(CR)(PDB ID:5DNA),在 N、C 和 NC 末端插入肽(CR-GrN、CR-GrC 和 CR-GrNC)对本地酶(CR-WT)进行改性,以评估位点特异性融合对电极结合的影响。评估了原生 CR 和合成 CR 相对于电极形貌的石墨表面结合活性,以确定酶-电极界面在高效电子导流方面的潜力。评估了特定位点肽融合和氨基酸定位对活性位点可用性/结合力和吸附/解吸能力的影响,以实现高效的二氧化碳氧化还原催化。通过结构、酶学和电化学特性研究了固体结合肽和石墨表面在直接电子传递方面的相互作用能力,以实现高效的二氧化碳电合成。总之,基于酶-电极复合物与肽修饰和石墨表面的交互能力,研究人员描绘了酶将二氧化碳还原为甲酸盐,从而实现生物电子学升级的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
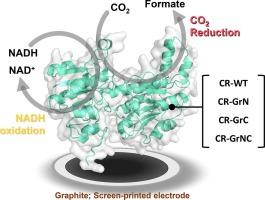
NADH-dependent CO2 reductase on graphite for capacitive electrocatalytic interfacing mediated by solid-binding peptide
NAD+/NADH-dependent CO2 reductase (CR) adapted from Candida methylica (E.C. 1.17.1.9) was introduced with a non-native graphite-specific peptide (Gr; IMVTESSDYSSY) as molecular binder to modify the native enzyme (CR-WT) with peptide insertion at N, C and NC terminus (CR-GrN, CR-GrC and CR-GrNC) to assess the influence of site-specific fusion on electrode binding. Graphite surface-binding activity relative to the electrode topography was evaluated for both native and synthetic CRs to establish the enzyme-electrode interfacing potentiality for efficient electron channelling. Impact of site-specific peptide fusion and amino-acids positioning was assessed for the active site binding availability and adsorption/desorption capability towards competent CO2-based redox catalysis. Solid-binding peptide and graphite surface interactive ability on direct electron transfer was studied with structural, enzymatic and electrochemical characterizations for efficient CO2 electrosynthesis. Overall, enzymatic CO2 reduction to formate based on interactive potentiality of enzyme-electrode complex with peptide modifications and graphite surface towards possibility of bioelectronics upscaling was depicted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioresource Technology
工程技术-能源与燃料
CiteScore
20.80
自引率
19.30%
发文量
2013
审稿时长
12 days
期刊介绍:
Bioresource Technology publishes original articles, review articles, case studies, and short communications covering the fundamentals, applications, and management of bioresource technology. The journal seeks to advance and disseminate knowledge across various areas related to biomass, biological waste treatment, bioenergy, biotransformations, bioresource systems analysis, and associated conversion or production technologies.
Topics include:
• Biofuels: liquid and gaseous biofuels production, modeling and economics
• Bioprocesses and bioproducts: biocatalysis and fermentations
• Biomass and feedstocks utilization: bioconversion of agro-industrial residues
• Environmental protection: biological waste treatment
• Thermochemical conversion of biomass: combustion, pyrolysis, gasification, catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


