通过碳化作用实现脱氧醇-核亲和剂偶联
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在亲核偶联反应中直接使用可广泛获得的醇类原料作为合成物是合成界的一个长期目标。由于 C-O 键形成的固有电子不匹配,利用醇的传统方法需要预先激活一个偶联剂。在这里,游离醇通过原位活化被用作碳位前体,扭转了其传统的亲核行为,避免了预官能化的需要。该研究描述了直接催化醇类脱氧偶联以实现选择性 C-O 异质偶联的过程。机理研究支持离散碳位中间体,它可以被各种简单的亲核物截获。此外,还展示了该方案在天然产品和复杂活性药物成分方面的应用。这种方法与多种亲核物兼容,只需一步就能构建 C-O、C-S、C-C 和 C-N 键,展示了这种醇活化平台的广泛适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
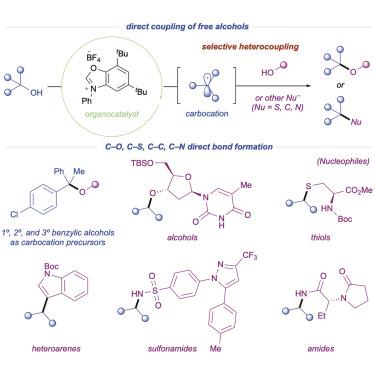
Deoxygenative alcohol–nucleophile coupling via carbocations
The direct employment of widely available alcohol feedstocks as synthons in nucleophilic couplings is a long-standing objective within the synthetic community. Traditional methods utilizing alcohols require the preactivation of one coupling partner due to the inherent mismatched electronics for C–O bond formation. Here, free alcohols are leveraged as carbocation precursors via in situ activation, reversing their traditional nucleophilic behavior and avoiding the need for prefunctionalization. The direct catalytic deoxygenative coupling of alcohols toward selective C–O heterocoupling is described. Mechanistic studies support the intermediacy of a discrete carbocation, which can be intercepted by a diverse array of simple nucleophiles. Application of this protocol toward natural products and complex active pharmaceutical ingredients is also demonstrated. The compatibility toward a large breadth of nucleophiles enables the construction of C–O, C–S, C–C, and C–N bonds in a single step, showcasing the broad applicability of this alcohol activation platform.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


