评估使用硼酸聚合物回收赤藓糖醇的效果
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
开发和评估下游阶段的新型材料和技术,是向更可持续和循环工艺过渡的主要挑战之一。赤藓糖醇作为一种低热量甜味剂以及制药和化妆品行业的基质,需求量很大。目前,赤藓糖醇是通过生物技术生产的,从复杂的培养液中回收赤藓糖醇是一项挑战。本研究评估了利用硼酸聚合物对赤藓糖醇的吸附从甘油和葡萄糖混合物中回收赤藓糖醇的情况。对 NaOH 与多元醇的比例和聚合物的质量等吸附参数进行了优化。在最佳条件下,吸附了赤藓糖醇初始质量的 71%,相当于 198 毫克赤藓糖醇/克聚合物的负载量。研究了由于与苯硼酸盐分子络合而产生的化学吸附和由于凝胶型聚合物的保留而产生的物理吸附。尽管物理吸附导致总体选择性下降,但由于络合作用,红藓醇的吸附选择性很高,分离因子高达 4。解吸策略包括洗涤和用 H2SO4 解吸,结果回收了 59% 的赤藓醇初始质量。凝胶型聚合物在酸性条件下收缩是解吸过程中多元醇回收的主要挑战。固态 11B 和 13C NMR 光谱用于研究吸附和解吸步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
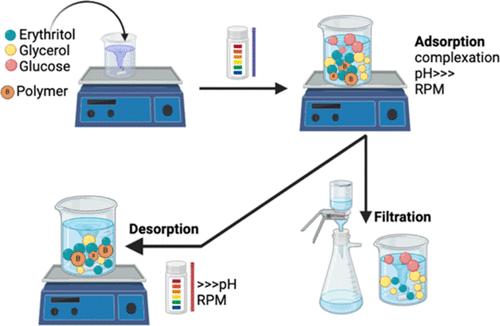
Assessment of the Recovery of Erythritol Using Boronic Acid Polymers
The development and assessment of novel materials and technologies in the downstream stages is one of the main challenges for the technological transition toward more sustainable and circular processes. Erythritol is in high demand as a low-calorie sweetener as well as a substrate in the pharmaceutical and cosmetic industries. Nowadays, erythritol is produced biotechnologically, and its recovery from complex cultivation broths poses a challenge. In this study, the recovery of erythritol from a mixture with glycerol and glucose using the adsorption of erythritol onto boronic acid polymers was evaluated. p-vinylphenylboronic acid was cross-linked by divinylbenzene; the polymer was characterized and used as an adsorbent. The adsorption parameters, such as NaOH-to-polyols ratio and mass of polymer, were optimized. Under the best conditions, 71% of the initial mass of erythritol was adsorbed, equivalent to a load of 198 mg of erythritol/g of polymer. Chemical adsorption owing to complexation with phenylboronate moieties and physical adsorption due to retention of the gel-type polymer were studied. Highly selective erythritol uptake owing to complexation with a separation factor of up to 4 was observed, though the contribution of the physical adsorption was responsible for a decrease in overall selectivity. The desorption strategies included washing and a desorption with H2SO4, resulting in the recovery of 59% of the initial mass of erythritol. Shrinkage of the gel-type polymer under acidic conditions was the major challenge in the recovery of the polyols during the desorption. Solid-state 11B and 13C NMR spectroscopy were used to examine the adsorption and desorption steps.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


