类胶质味觉细胞介导外周甜味适应的细胞间模式
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
一般来说,味觉对长时间甜味刺激的敏感性会降低,这被称为甜味适应。然而,其机理图谱仍不完整。在这里,我们报告了神经胶质样 I 型细胞通过与化学感觉 II 型细胞的细胞间串扰提供了一种独特的甜味适应模式。利用基于微流控的舌内成像系统,我们发现甜味适应是通过 II 型细胞到味觉传入神经的突触传导来促进的,而 I 型细胞则表现出时间延迟和延长的活动。我们发现 I 型细胞通过 P2RY2 接收相邻 II 型细胞的嘌呤能输入,并为甜味的突触传导提供抑制反馈。与我们在细胞水平上的发现一致,I型细胞的嘌呤能激活减弱了甜味舔食行为,P2RY2基因敲除小鼠的适应行为减慢。我们的研究突显了一种隐蔽的细胞间甜味适应模式,它可能有助于对延长的甜味进行有效编码。本文章由计算机程序翻译,如有差异,请以英文原文为准。
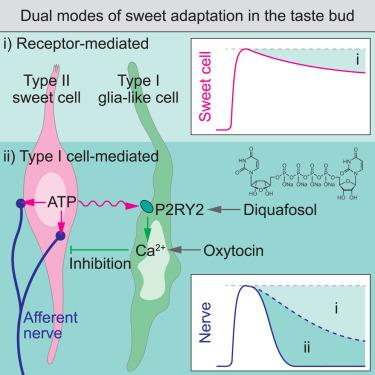
Glia-like taste cells mediate an intercellular mode of peripheral sweet adaptation
The sense of taste generally shows diminishing sensitivity to prolonged sweet stimuli, referred to as sweet adaptation. Yet, its mechanistic landscape remains incomplete. Here, we report that glia-like type I cells provide a distinct mode of sweet adaptation via intercellular crosstalk with chemosensory type II cells. Using the microfluidic-based intravital tongue imaging system, we found that sweet adaptation is facilitated along the synaptic transduction from type II cells to gustatory afferent nerves, while type I cells display temporally delayed and prolonged activities. We identified that type I cells receive purinergic input from adjacent type II cells via P2RY2 and provide inhibitory feedback to the synaptic transduction of sweet taste. Aligning with our cellular-level findings, purinergic activation of type I cells attenuated sweet licking behavior, and P2RY2 knockout mice showed decelerated adaptation behavior. Our study highlights a veiled intercellular mode of sweet adaptation, potentially contributing to the efficient encoding of prolonged sweetness.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


