ACSS2 发挥乳酰-CoA 合成酶的作用,并与 KAT2A 相互配合,发挥乳酰转移酶的功能,促进组蛋白乳酰化和肿瘤免疫逃避
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
乳酰辅酶 A(CoA)依赖的组蛋白赖氨酸乳酰化影响基因表达,并在生物过程中发挥着重要作用。然而,哺乳动物的乳酰辅酶 A 合成酶及其对组蛋白乳酰化的调控尚未被发现。在这里,我们证明了表皮生长因子受体(EGFR)激活会诱导细胞外信号调节激酶(ERK)介导的乙酰-CoA 合成酶 2(ACSS2)的 S267 磷酸化及其随后的核转位和与赖氨酸乙酰转移酶 2A (KAT2A)形成复合物。重要的是,ACSS2 具有真正的乳酰-CoA 合成酶功能,可将乳酸转化为乳酰-CoA,而乳酰-CoA 可与 KAT2A 结合,这一点已通过共晶体结构分析得到证实。因此,KAT2A 可作为乳酰转移酶对组蛋白 H3 进行乳酰化,从而导致 Wnt/β-catenin、NF-κB 和 PD-L1 的表达以及脑肿瘤的生长和免疫逃避。ACSS2-KAT2A 相互作用阻断肽和抗 PD-1 抗体的联合治疗可产生相加的肿瘤抑制效果。这些发现揭示了 ACSS2 和 KAT2A 分别是迄今为止尚未发现的乳酰-CoA 合成酶和乳酰转移酶,以及 ACSS2-KAT2A 偶联在基因表达和肿瘤发生中的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
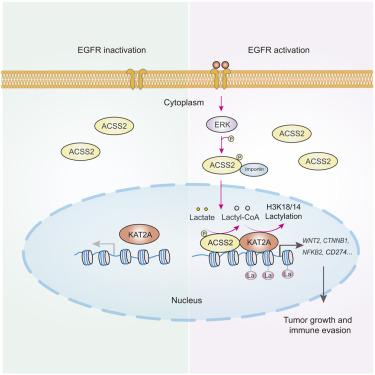
ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion
Lactyl-coenzyme A (CoA)-dependent histone lysine lactylation impacts gene expression and plays fundamental roles in biological processes. However, mammalian lactyl-CoA synthetases and their regulation of histone lactylation have not yet been identified. Here, we demonstrate that epidermal growth factor receptor (EGFR) activation induces extracellular signal-regulated kinase (ERK)-mediated S267 phosphorylation of acetyl-CoA synthetase 2 (ACSS2) and its subsequent nuclear translocation and complex formation with lysine acetyltransferase 2A (KAT2A). Importantly, ACSS2 functions as a bona fide lactyl-CoA synthetase and converts lactate to lactyl-CoA, which binds to KAT2A as demonstrated by a co-crystal structure analysis. Consequently, KAT2A acts as a lactyltransferase to lactylate histone H3, leading to the expression of Wnt/β-catenin, NF-κB, and PD-L1 and brain tumor growth and immune evasion. A combination treatment with an ACSS2-KAT2A interaction-blocking peptide and an anti-PD-1 antibody induces an additive tumor-inhibitory effect. These findings uncover ACSS2 and KAT2A as hitherto unidentified lactyl-CoA synthetase and lactyltransferase, respectively, and the significance of the ACSS2-KAT2A coupling in gene expression and tumor development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


